Neuroprotective effects of vinpocetine, as a phosphodiesterase 1 inhibitor, on long-term potentiation in a rat model of Alzheimer's disease
- PMID: 36927298
- PMCID: PMC10018848
- DOI: 10.1186/s12868-023-00790-8
Neuroprotective effects of vinpocetine, as a phosphodiesterase 1 inhibitor, on long-term potentiation in a rat model of Alzheimer's disease
Abstract
Background: Vinpocetine (Vin) is known as a phosphodiesterase 1 inhibitor (PDE1-I) drug with multilateral effects, including antioxidant and anti-inflammatory activity. In this research, we investigated the neuroprotective and therapeutic effects of Vin through hippocampal synaptic plasticity on a rat's model of Alzheimer's disease (AD) induced by an intracerebroventricular (ICV) injection of beta-amyloid (Aβ).
Methods: Sixty adult male Wistar rats were randomly divided into six groups: 1. control, 2. sham, 3. Aβ, 4. pretreatment (Vin + Aβ): Vin (4 mg/kg, gavage) for 30 days and then, inducing an AD model by an ICV injection of Aβ(1-42), 5. treatment (Aβ + Vin): inducing an AD model and then receiving Vin for 30 days by gavage, and 7. pretreatment + treatment (Vin + Aβ + Vin): receiving Vin by gavage for 30 days before and 30 days after the induction of an AD model. After these procedures, via stereotaxic surgery, the stimulating electrodes were placed at the perforant pathway (PP) and the recording electrodes were implanted in the dentate gyrus.
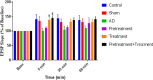
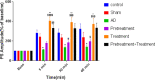
Results: Excitatory postsynaptic potential (EPSP) slope and population spike (PS) amplitude in the Aβ group meaningfully diminished compared to the control group after the induction of long-term potentiation (LTP).
Conclusions: Vin could significantly prevent the Aβ effects on LTP. It can be concluded that pretreatment and treatment with Vin can be neuroprotective against harmful consequences of Aβ on hippocampal synaptic plasticity.
Keywords: Alzheimer’s disease; Beta-amyloid; Hippocampus; Long-term potentiation; Phosphodiesterase1 inhibitor; Vinpocetine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
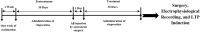


References
-
- Leslie RA. Imaging Alzheimer's disease in vivo: not so ‘implaque-able’anymore. Trends Neurosci. 2002;25:232–233. doi: 10.1016/S0166-2236(02)02160-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

