The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades
- PMID: 36927623
- PMCID: PMC10018889
- DOI: 10.1186/s13045-023-01409-5
The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades
Abstract
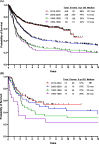
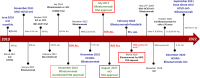
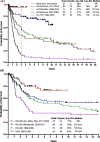
Progress in the research and therapy of adult acute lymphoblastic leukemia (ALL) is accelerating. This analysis summarizes the data derived from the clinical trials conducted at MD Anderson between 1985 and 2022 across ALL subtypes. In Philadelphia chromosome-positive ALL, the addition of BCR::ABL1 tyrosine kinase inhibitors (TKIs) to intensive chemotherapy since 2000, improved outcomes. More recently, a chemotherapy-free regimen with blinatumomab and ponatinib resulted in a complete molecular remission rate of 85% and an estimated 3-year survival rate of 90%, potentially reducing the role of, and need for allogeneic stem cell transplantation (SCT) in remission. In younger patients with pre-B Philadelphia chromosome-negative ALL, the integration of blinatumomab and inotuzumab into the frontline therapy has improved the estimated 3-year survival rate to 85% across all risk categories. Our future strategy is to evaluate the early integration of both immunotherapy agents, inotuzumab and blinatumomab, with low-dose chemotherapy (dose-dense mini-Hyper-CVD-inotuzumab-blinatumomab) into the frontline setting followed by CAR T cells consolidation in high-risk patients, without any further maintenance therapy. In older patients, using less intensive chemotherapy (mini-Hyper-CVD) in combination with inotuzumab and blinatumomab has improved the 5-year survival rate to 50%. Among patients ≥ 65-70 years, the mortality in complete remission (CR) is still high and is multifactorial (old age, death in CR with infections, development of myelodysplastic syndrome or acute myeloid leukemia). A chemotherapy-free regimen with inotuzumab and blinatumomab is being investigated. The assessment of measurable residual disease (MRD) by next-generation sequencing (NGS) is superior to conventional assays, with early MRD negativity by NGS being associated with the best survival. We anticipate that the future therapy in B-ALL will involve less intensive and shorter chemotherapy regimens in combination with agents targeting CD19 (blinatumomab), CD20, and CD22 (inotuzumab). The optimal timing and use of CAR T cells therapy may be in the setting of minimal disease, and future trials will assess the role of CAR T cells as a consolidation among high-risk patients to replace allogeneic SCT. In summary, the management of ALL has witnessed significant progress during the past four decades. Novel combination regimens including newer-generation BCR::ABL1 TKIs and novel antibodies are questioning the need and duration of intensive chemotherapy and allogeneic SCT.
Keywords: Blinatumomab; CAR T cell; Chemotherapy-free; Cure; Evolution; Inotuzumab; Ponatinib; Targeted therapies; Tyrosine kinase inhibitors.
© 2023. The Author(s).
Conflict of interest statement
EJ received research grants from Abbvie, adaptive biotechnologies, Amgen, Pfizer, and Takeda; and consultancy fees from Abbvie, adaptive biotechnologies, Amgen, BMS, Genentech, Incyte, Novartis, Pfizer, and Takeda. NJS received research grants from Takeda Oncology, Astellas Pharma Inc., Xencor and Stemline Therapeutics; consultancy fees from Pfizer Inc. and Jazz Pharmaceuticals; and honoraria from Novartis, Amgen, Sanofi, and BeiGene. HK received research grants from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, Novartis, Pfizer; and honoraria from AbbVie, Amgen, Aptitude Health, Ascentage, Astellas Health, Astra Zeneca, Ipsen, Pharmaceuticals, KAHR Medical Ltd, NOVA Research, Novartis, Pfizer, Precision Biosciences, Taiho Pharmaceutical Canada. FGH, MAW, NJ, and FR declare no competing interests.
Figures


References
-
- Jabbour E, Thomas D, Cortes J, Kantarjian HM, O'Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia. Cancer. 2010;116(10):2290–2300. - PubMed
-
- Kantarjian H, Andreeff M, Keating M, Kornblau S, Ferrajoli A, Verstovsek S, et al. Update of the modified hyper-CVAD regimen with or without rituximab in newly diagnosed adult acute lymphocytic leukemia (ALL) Blood. 2005;106(11):1831. doi: 10.1182/blood.V106.11.1831.1831. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

