Outcomes with Finerenone in Participants with Stage 4 CKD and Type 2 Diabetes: A FIDELITY Subgroup Analysis
- PMID: 36927680
- PMCID: PMC10278789
- DOI: 10.2215/CJN.0000000000000149
Outcomes with Finerenone in Participants with Stage 4 CKD and Type 2 Diabetes: A FIDELITY Subgroup Analysis
Abstract
Background: Patients with stage 4 CKD and type 2 diabetes have limited treatment options to reduce their persistent cardiovascular and kidney risk. In Finerenone in Chronic Kidney Disease and Type 2 Diabetes (FIDELITY), a prespecified pooled analysis of Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD), finerenone improved heart-kidney outcomes in participants with CKD and type 2 diabetes.
Methods: This FIDELITY subgroup analysis investigated the effects of finerenone in participants with stage 4 CKD (eGFR <30 ml/min per 1.73 m 2 ). Efficacy outcomes included a cardiovascular composite (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (kidney failure, sustained ≥57% decrease in eGFR from baseline, or kidney disease death).
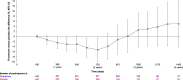
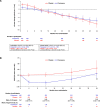
Results: Of 13,023 participants, 890 (7%) had stage 4 CKD. The hazard ratio for risk of cardiovascular composite outcome with finerenone versus placebo was 0.78 (95% confidence interval, 0.57 to 1.07). The kidney composite outcome proportional hazards assumption was not met for the overall study period, with a protective effect only shown up to 2 years, after which the direction of association was inconsistent, and an observed loss of precision over time incurred on finerenone versus placebo risk differences. Nonetheless, albuminuria and rate of eGFR decline were consistently reduced with finerenone versus placebo. Adverse events were balanced between treatment arms. Hyperkalemia was the most common adverse event reported (stage 4 CKD: 26% and 13% for finerenone versus placebo, respectively); however, the incidence of hyperkalemia leading to permanent discontinuation was low (stage 4 CKD: 3% and 2% for finerenone versus placebo, respectively).
Conclusions: The cardiovascular benefits and safety profile of finerenone in participants with stage 4 CKD were consistent with the overall FIDELITY population; this was also the case for albuminuria and the rate of eGFR decline. The effects on the composite kidney outcome were not consistent over time.
Trial registration: ClinicalTrials.gov NCT02540993 NCT02545049.
Copyright © 2023 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of American Society of Nephrology.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

