Maternal hyperglycemia inhibits pulmonary vasculogenesis during mouse fetal lung development by promoting GβL Ubiquitination-dependent mammalian target of Rapamycin assembly
- PMID: 36927703
- PMCID: PMC10021989
- DOI: 10.1186/s13098-022-00974-y
Maternal hyperglycemia inhibits pulmonary vasculogenesis during mouse fetal lung development by promoting GβL Ubiquitination-dependent mammalian target of Rapamycin assembly
Abstract
Background: Gestational diabetes mellitus (GDM) is associated with retarded lung development and poor lung health in offspring. Mammalian target of rapamycin (mTOR) is a key regulator of vasculogenesis and angiogenesis. The aim of this study was to investigate the role mTOR plays in pulmonary vasculogenesis during fetal lung development under maternal hyperglycemia.
Methods: First, GDM was induced via streptozotocin injection in pregnant C57BL/6 mice before the radial alveolar count (RAC) in the fetal lungs was assessed using hematoxylin and eosin staining. The angiogenic ability of the cultured primary mouse fetal lung endothelial cells (MFLECs) was then assessed using the tube formation assay technique, while western blot and real-time polymerase chain reaction were performed to determine the expression of mTOR, regulatory-associated protein of mTOR (Raptor), rapamycin-insensitive companion of mTOR (Rictor), stress-activated protein kinase interacting protein 1 (Sin1), G protein beta subunit-like protein (GβL), Akt, tumor necrosis receptor associated factor-2 (TRAF2), and OTU deubiquitinase 7B (OTUD7B) in both the fetal lung tissues and the cultured MFLECs. Immunoprecipitation assays were conducted to evaluate the status of GβL-ubiquitination and the association between GβL and mTOR, Raptor, Rictor, and Sin1 in the cultured MFLECs.
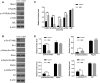
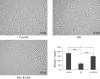
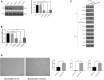
Results: The GDM fetal lungs exhibited a decreased RAC and reduced expression of von Willebrand factor, CD31, and microvessel density. The high glucose level reduced the tube formation ability in the MFLECs, with the mTOR, p-mTOR, p-Raptor, and TRAF2 expression upregulated and the p-Rictor, p-Sin1, p-Akt, and OTUD7B expression downregulated in both the GDM fetal lungs and the high-glucose-treated MFLECs. Meanwhile, GβL-ubiquitination was upregulated in the high-glucose-treated MFLECs along with an increased GβL/Raptor association and decreased GβL/Rictor and GβL/Sin1 association. Furthermore, TRAF2 knockdown inhibited the high-glucose-induced GβL-ubiquitination and GβL/Raptor association and restored the tube formation ability of the MFLECs.
Conclusion: Maternal hyperglycemia inhibits pulmonary vasculogenesis during fetal lung development by promoting GβL-ubiquitination-dependent mTORC1 assembly.
Keywords: Gestational diabetes mellitus; Pulmonary development; Ubiquitination; mTORC1; mTORC2.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Anderson JL, Waller DK, Canfield MA, Shaw GM, Watkins ML, Werler MM. Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology. 2005;16(1):87–92. doi: 10.1097/01.ede.0000147122.97061.bb. - DOI - PubMed
-
- Azad MB, Moyce BL, Guillemette L, Pascoe CD, Wicklow B, McGavock JM, Halayko AJ, Dolinsky VW. Diabetes in pregnancy and lung health in offspring: developmental origins of respiratory disease. Paediatr Respir Rev. 2017;21:19–26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

