Deep Brain Stimulation of the Anterior Nucleus of the Thalamus in Drug-Resistant Epilepsy in the MORE Multicenter Patient Registry
- PMID: 36927882
- PMCID: PMC10159763
- DOI: 10.1212/WNL.0000000000206887
Deep Brain Stimulation of the Anterior Nucleus of the Thalamus in Drug-Resistant Epilepsy in the MORE Multicenter Patient Registry
Abstract
Background and objectives: The efficacy of deep brain stimulation of the anterior nucleus of the thalamus (ANT DBS) in patients with drug-resistant epilepsy (DRE) was demonstrated in the double-blind Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy randomized controlled trial. The Medtronic Registry for Epilepsy (MORE) aims to understand the safety and longer-term effectiveness of ANT DBS therapy in routine clinical practice.
Methods: MORE is an observational registry collecting prospective and retrospective clinical data. Participants were at least 18 years old, with focal DRE recruited across 25 centers from 13 countries. They were followed for at least 2 years in terms of seizure frequency (SF), responder rate (RR), health-related quality of life (Quality of Life in Epilepsy Inventory 31), depression, and safety outcomes.
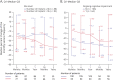
Results: Of the 191 patients recruited, 170 (mean [SD] age of 35.6 [10.7] years, 43% female) were implanted with DBS therapy and met all eligibility criteria. At baseline, 38% of patients reported cognitive impairment. The median monthly SF decreased by 33.1% from 15.8 at baseline to 8.8 at 2 years (p < 0.0001) with 32.3% RR. In the subgroup of 47 patients who completed 5 years of follow-up, the median monthly SF decreased by 55.1% from 16 at baseline to 7.9 at 5 years (p < 0.0001) with 53.2% RR. High-volume centers (>10 implantations) had 42.8% reduction in median monthly SF by 2 years in comparison with 25.8% in low-volume center. In patients with cognitive impairment, the reduction in median monthly SF was 26.0% by 2 years compared with 36.1% in patients without cognitive impairment. The most frequently reported adverse events were changes (e.g., increased frequency/severity) in seizure (16%), memory impairment (patient-reported complaint, 15%), depressive mood (patient-reported complaint, 13%), and epilepsy (12%). One definite sudden unexpected death in epilepsy case was reported.
Discussion: The MORE registry supports the effectiveness and safety of ANT DBS therapy in a real-world setting in the 2 years following implantation.
Classification of evidence: This study provides Class IV evidence that ANT DBS reduces the frequency of seizures in patients with drug-resistant focal epilepsy.
Trial registration information: MORE ClinicalTrials.gov Identifier: NCT01521754, first posted on January 31, 2012.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
J. Peltola has participated in clinical trials for Eisai, UCB, and Bial; received research grants from Eisai, Medtronic, UCB, and Livanova; received speaker honoraria from LivaNova, Eisai, Medtronic, Orion Pharma, and UCB; received support for travel to congresses from LivaNova, Eisai, Medtronic, and UCB; and participated in advisory boards for Arvelle, Novartis, LivaNova, Eisai, Medtronic, UCB, and GW Pharma. A.J. Colon received speaker honoraria from Medtronic. J. Pimentel received occasionally fees from Medtronic for speeches, travel meetings and collecting data for the MORE registry, and courses. V.A. Coenen received honoraria for talks from Boston Scientific, USA. He has received grants for clinical trials (IITs) from Boston Scientific, USA, and Medtronic, USA. He receives an ongoing collaborative grant from BrainLab (Munich, Germany). V.A. Coenen is scientific advisor for CereGate (Hamburg) and Cortec (Freiburg). A. Gil-Nagel received grants or honoraria as speaker or advisory board from Bial, Biocodex, Eisai, Stoke Therapeutics, GW Pharma, Esteve, UCB Pharma, Zogenix, and Arvelle Therapeutics. A. Gonçalves Ferreira declares Medtronic support to his participation in different scientific events including being member of the MORE Steering Committee. K. Lehtimäki received speaker or consultancy fees from Medtronic. P. Ryvlin has received speaker or consultant fees from UCB Pharma, GW Pharmaceutical, Eisai, Arvelle, Livanova, and Medtronic. R.S. Taylor is a paid consultant for Medtronic. However, he received no payment associated with preparation/contribution to this manuscript. L. Ackermans reports no disclosures relevant to the manuscript. J. Ardesch reports no disclosures relevant to the manuscript; she is a member of a data safety monitoring board, nonprofit. C. Bentes reports that the Reference Centre for Refractory Epilepsy from Hospital de Santa Maria –Centro Hospitalar Lisboa Norte received fees from Medtronic in January 2021 for the educational course: Curso Luso Brasileiro DBS em Epilepsia. M. Bosak received honoraria for publications for and participation in advisory meetings from Sanofi, honoraria for lectures, travel expenses, and conference fees from Sanofi, Adamed, Teva Pharmaceutical, Neuraxpharm, Glenmark, UCB Pharma, and Zentiva. J.G. Burneo is the Jack Cowin endowed chair in Epilepsy Research at Western University. C. Chamadoira, C.E. Elger, L. Erőss, D. Fabo, H. Faulkner, J. Gawlowicz, A. Gharabaghi, and M. Iacoangeli report no disclosures relevant to the manuscript. J. Janszky received consultation fees from Hungarian subsidiaries of UCB, Richter, and Gerot. Regarding this study, the author did not receive any corporate funding and receives research support from the Hungarian Brain Research Program (2017-1.2.1-NKP-2017-00002), NKFIH EFOP-3.6.2-16-2017-00008 government-based funds. S. Järvenpää received study grants from the Maire Taponen Foundation, Finnish Epilepsy Research Foundation, Finnish Medical Foundation, and Instrumentarium Science Foundation, none of which are relevant to the manuscript. E. Kaufmann has received speaker honoraria from Medtronic. K.H. Kho, E. Kumlien, H. Laufs, C. Lettieri, P. Linhares, S. Noachtar, A. Parrent, and E. Pataraia report no disclosures relevant to the manuscript. N.K. Patel reports no disclosures relevant to the manuscript. He is clinical director at Bioinduction Ltd. A.R. Peralta reports that Centro de Referência de Epilepsia Refractária, of which A.R. Peralta is a member, received speaker honoraria from Medtronic. A. Rácz received fees as a speaker from UCB Pharma. A. Rainha Campos received a travel grant and speaker honorarium from Medtronic. R. Rego received advisory honoraria from Medtronic. R.A. Ricciuti reports no disclosures relevant to the manuscript. S. Rona discloses compensation for data collection in the MORE study. R.P.W. Rouhl reports no disclosures relevant to the manuscript. A. Schulze-Bonhage has received research funding from BIAL, BMBF, DFG, EU, NIH, Precisis, and UNEEG. He has received honoraria for advice or lectures from Arvelle, BIAL, EISAI, GW, and UCB. R. Schuurman acts as consultant for Medtronic on educational matters. M. Sprengers, A. Sufianov, and Y. Temel report no disclosures relevant to the manuscript. T. Theys holds a learning chair Neuromodulation, an endowment from Medtronic. W. Van Paesschen, D. Van Roost, and R. Vaz report no disclosures relevant to the manuscript. K. Vonck has been funded by the Bijzonder Onderzoeksfonds (BOF) of Ghent University and received funding from the Geneeskundige Stichting Koningin Elisabeth (GSKE) for Neuroscience. K. Vonckt is also a consultant for Synergia Medical and Livanova and has received free devices for research studies in normal volunteers and preclinical studies from Cerbomed. L. Wagner and J. Zwemmer report no disclosures relevant to the manuscript. A. Abouihia was a Medtronic Employee at the time of performing the analyses and development of the manuscript. T.C. Brionne is a Medtronic Employee. F. Gielen is a Medtronic Employee. P.A.J.M. Boon received speaker and consultancy fees from LivaNova, Medtronic, UCB Pharma, and Eisai. Go to
Figures

Comment in
-
What Should We Expect for Real-World Outcomes of Deep Brain Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy?Neurology. 2023 May 2;100(18):845-846. doi: 10.1212/WNL.0000000000201455. Epub 2023 Mar 20. Neurology. 2023. PMID: 36941073 No abstract available.
-
Is MORE better? Accumulating Evidence for ANT DBS in Epilepsy.Epilepsy Curr. 2023 Sep 4;23(6):357-359. doi: 10.1177/15357597231197086. eCollection 2023 Nov-Dec. Epilepsy Curr. 2023. PMID: 38269353 Free PMC article.
References
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. - PubMed
-
- Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2-3):310-318. - PubMed
-
- Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA. 2015;313(3):285-293. - PubMed
-
- Gooneratne IK, Green AL, Dugan P, et al. . Comparing neurostimulation technologies in refractory focal-onset epilepsy. J Neurol Neurosurg Psychiatry. 2016;87(11):1174-1182. - PubMed
-
- Fisher R, Salanova V, Witt T, et al. . Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
