Pharmacogenomics: Driving Personalized Medicine
- PMID: 36927888
- PMCID: PMC10289244
- DOI: 10.1124/pharmrev.122.000810
Pharmacogenomics: Driving Personalized Medicine
Abstract
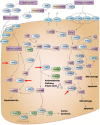
Personalized medicine tailors therapies, disease prevention, and health maintenance to the individual, with pharmacogenomics serving as a key tool to improve outcomes and prevent adverse effects. Advances in genomics have transformed pharmacogenetics, traditionally focused on single gene-drug pairs, into pharmacogenomics, encompassing all "-omics" fields (e.g., proteomics, transcriptomics, metabolomics, and metagenomics). This review summarizes basic genomics principles relevant to translation into therapies, assessing pharmacogenomics' central role in converging diverse elements of personalized medicine. We discuss genetic variations in pharmacogenes (drug-metabolizing enzymes, drug transporters, and receptors), their clinical relevance as biomarkers, and the legacy of decades of research in pharmacogenetics. All types of therapies, including proteins, nucleic acids, viruses, cells, genes, and irradiation, can benefit from genomics, expanding the role of pharmacogenomics across medicine. Food and Drug Administration approvals of personalized therapeutics involving biomarkers increase rapidly, demonstrating the growing impact of pharmacogenomics. A beacon for all therapeutic approaches, molecularly targeted cancer therapies highlight trends in drug discovery and clinical applications. To account for human complexity, multicomponent biomarker panels encompassing genetic, personal, and environmental factors can guide diagnosis and therapies, increasingly involving artificial intelligence to cope with extreme data complexities. However, clinical application encounters substantial hurdles, such as unknown validity across ethnic groups, underlying bias in health care, and real-world validation. This review address the underlying science and technologies germane to pharmacogenomics and personalized medicine, integrated with economic, ethical, and regulatory issues, providing insights into the current status and future direction of health care. SIGNIFICANCE STATEMENT: Personalized medicine aims to optimize health care for the individual patients with use of predictive biomarkers to improve outcomes and prevent adverse effects. Pharmacogenomics drives biomarker discovery and guides the development of targeted therapeutics. This review addresses basic principles and current trends in pharmacogenomics, with large-scale data repositories accelerating medical advances. The impact of pharmacogenomics is discussed, along with hurdles impeding broad clinical implementation, in the context of clinical care, ethics, economics, and regulatory affairs.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

