Inhibition of ERK1/2 signaling prevents bone marrow fibrosis by reducing osteopontin plasma levels in a myelofibrosis mouse model
- PMID: 36928007
- PMCID: PMC10169646
- DOI: 10.1038/s41375-023-01867-3
Inhibition of ERK1/2 signaling prevents bone marrow fibrosis by reducing osteopontin plasma levels in a myelofibrosis mouse model
Abstract
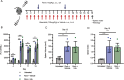
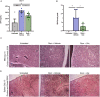
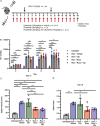
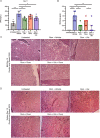
Clonal myeloproliferation and development of bone marrow (BM) fibrosis are the major pathogenetic events in myelofibrosis (MF). The identification of novel antifibrotic strategies is of utmost importance since the effectiveness of current therapies in reverting BM fibrosis is debated. We previously demonstrated that osteopontin (OPN) has a profibrotic role in MF by promoting mesenchymal stromal cells proliferation and collagen production. Moreover, increased plasma OPN correlated with higher BM fibrosis grade and inferior overall survival in MF patients. To understand whether OPN is a druggable target in MF, we assessed putative inhibitors of OPN expression in vitro and identified ERK1/2 as a major regulator of OPN production. Increased OPN plasma levels were associated with BM fibrosis development in the Romiplostim-induced MF mouse model. Moreover, ERK1/2 inhibition led to a remarkable reduction of OPN production and BM fibrosis in Romiplostim-treated mice. Strikingly, the antifibrotic effect of ERK1/2 inhibition can be mainly ascribed to the reduced OPN production since it could be recapitulated through the administration of anti-OPN neutralizing antibody. Our results demonstrate that OPN is a novel druggable target in MF and pave the way to antifibrotic therapies based on the inhibition of ERK1/2-driven OPN production or the neutralization of OPN activity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

