CDX-2 expression correlates with clinical outcomes in MSI-H metastatic colorectal cancer patients receiving immune checkpoint inhibitors
- PMID: 36928082
- PMCID: PMC10020482
- DOI: 10.1038/s41598-023-31538-3
CDX-2 expression correlates with clinical outcomes in MSI-H metastatic colorectal cancer patients receiving immune checkpoint inhibitors
Abstract
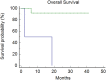
Immune checkpoint inhibitors (ICIs) showed efficacy in metastatic colorectal cancer (mCRC) with mismatch-repair deficiency or high microsatellite instability (dMMR-MSI-H). Unfortunately, a patient's subgroup did not benefit from immunotherapy. Caudal-related homeobox transcription factor 2 (CDX-2) would seem to influence immunotherapy's sensitivity, promoting the chemokine (C-X-C motif) ligand 14 (CXCL14) expression. Therefore, we investigated CDX-2 role as a prognostic-predictive marker in patients with mCRC MSI-H. We retrospectively collected data from 14 MSI-H mCRC patients treated with ICIs between 2019 and 2021. The primary endpoint was the 12-month progression-free-survival (PFS) rate. The secondary endpoints were overall survival (OS), PFS, objective response rate (ORR), and disease control rate (DCR). The PFS rate at 12 months was 81% in CDX-2 positive patients vs 0% in CDX-2 negative patients (p = 0.0011). The median PFS was not reached (NR) in the CDX-2 positive group versus 2.07 months (95%CI 2.07-10.8) in CDX-2 negative patients (p = 0.0011). Median OS was NR in CDX-2-positive patients versus 2.17 months (95% Confidence Interval [CI] 2.17-18.7) in CDX2-negative patients (p = 0.026). All CDX-2-positive patients achieved a disease response, one of them a complete response. Among CDX-2-negative patients, one achieved stable disease, while the other progressed rapidly (ORR: 100% vs 0%, p = 0.0005; DCR: 100% vs 50%, p = 0.02). Twelve patients received 1st-line pembrolizumab (11 CDX-2 positive and 1 CDX-2 negative) not reaching median PFS, while two patients (1 CDX-2 positive and 1 CDX-2 negative) received 3rd-line pembrolizumab reaching a median PFS of 10.8 months (95% CI, 10.8-12.1; p = 0.036). Although our study reports results on a small population, the prognostic role of CDX-2 in CRC seems confirmed and could drive a promising predictive role in defining the population more sensitive to immunotherapy treatment. Modulating the CDX-2/CXCL14 axis in CDX-2-negative patients could help overcome primary resistance to immunotherapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Comparative Effectiveness of Immune Checkpoint Inhibitors vs Chemotherapy in Patients With Metastatic Colorectal Cancer With Measures of Microsatellite Instability, Mismatch Repair, or Tumor Mutational Burden.JAMA Netw Open. 2023 Jan 3;6(1):e2252244. doi: 10.1001/jamanetworkopen.2022.52244. JAMA Netw Open. 2023. PMID: 36689222 Free PMC article.
-
Tumour mutational burden as a biomarker in patients with mismatch repair deficient/microsatellite instability-high metastatic colorectal cancer treated with immune checkpoint inhibitors.Eur J Cancer. 2023 Jul;187:15-24. doi: 10.1016/j.ejca.2023.03.029. Epub 2023 Mar 31. Eur J Cancer. 2023. PMID: 37099945
-
Early treatment discontinuation in patients with deficient mismatch repair or microsatellite instability high metastatic colorectal cancer receiving immune checkpoint inhibitors.J Immunother Cancer. 2025 Jan 4;13(1):e010424. doi: 10.1136/jitc-2024-010424. J Immunother Cancer. 2025. PMID: 39755579 Free PMC article.
-
Outcomes Following Immune Checkpoint Inhibitor Treatment of Patients With Microsatellite Instability-High Cancers: A Systematic Review and Meta-analysis.JAMA Oncol. 2020 Jul 1;6(7):1068-1071. doi: 10.1001/jamaoncol.2020.1046. JAMA Oncol. 2020. PMID: 32407439 Free PMC article.
-
Clinical benefits of PD-1/PD-L1 inhibitors in patients with metastatic colorectal cancer: a systematic review and meta-analysis.World J Surg Oncol. 2022 Mar 24;20(1):93. doi: 10.1186/s12957-022-02549-7. World J Surg Oncol. 2022. PMID: 35331250 Free PMC article.
Cited by
-
Prediction of Response to Anti-Angiogenic Treatment for Advanced Colorectal Cancer Patients: From Biological Factors to Functional Imaging.Cancers (Basel). 2024 Mar 30;16(7):1364. doi: 10.3390/cancers16071364. Cancers (Basel). 2024. PMID: 38611042 Free PMC article. Review.
-
Case report: Efficacy of immunotherapy as conversion therapy in dMMR/MSI-H colorectal cancer: a case series and review of the literature.Front Immunol. 2024 Feb 1;15:1352262. doi: 10.3389/fimmu.2024.1352262. eCollection 2024. Front Immunol. 2024. PMID: 38361927 Free PMC article. Review.
-
CD44: A New Prognostic Marker in Colorectal Cancer?Cancers (Basel). 2024 Apr 19;16(8):1569. doi: 10.3390/cancers16081569. Cancers (Basel). 2024. PMID: 38672650 Free PMC article. Review.
-
Advances in Precision Medicine Approaches for Colorectal Cancer: From Molecular Profiling to Targeted Therapies.ACS Pharmacol Transl Sci. 2024 Mar 19;7(4):967-990. doi: 10.1021/acsptsci.4c00008. eCollection 2024 Apr 12. ACS Pharmacol Transl Sci. 2024. PMID: 38633600 Free PMC article. Review.
-
Recent advances and challenges in colorectal cancer: From molecular research to treatment.World J Gastroenterol. 2025 Jun 7;31(21):106964. doi: 10.3748/wjg.v31.i21.106964. World J Gastroenterol. 2025. PMID: 40538516 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

