Iron homeostasis governs erythroid phenotype in polycythemia vera
- PMID: 36928379
- PMCID: PMC10646816
- DOI: 10.1182/blood.2022016779
Iron homeostasis governs erythroid phenotype in polycythemia vera
Abstract
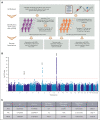
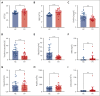
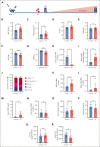
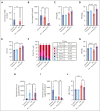
Polycythemia vera (PV) is a myeloproliferative neoplasm driven by activating mutations in JAK2 that result in unrestrained erythrocyte production, increasing patients' hematocrit and hemoglobin concentrations, placing them at risk of life-threatening thrombotic events. Our genome-wide association study of 440 PV cases and 403 351 controls using UK Biobank data showed that single nucleotide polymorphisms in HFE known to cause hemochromatosis are highly associated with PV diagnosis, linking iron regulation to PV. Analysis of the FinnGen dataset independently confirmed overrepresentation of homozygous HFE variants in patients with PV. HFE influences the expression of hepcidin, the master regulator of systemic iron homeostasis. Through genetic dissection of mouse models of PV, we show that the PV erythroid phenotype is directly linked to hepcidin expression: endogenous hepcidin upregulation alleviates erythroid disease whereas hepcidin ablation worsens it. Furthermore, we demonstrate that in PV, hepcidin is not regulated by expanded erythropoiesis but is likely governed by inflammatory cytokines signaling via GP130-coupled receptors. These findings have important implications for understanding the pathophysiology of PV and offer new therapeutic strategies for this disease.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: U.S. is a full-time employee of Silence Therapeutics GmbH and has stock options. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Manipulating hepcidin in polycythemia vera.Blood. 2023 Jun 29;141(26):3132-3134. doi: 10.1182/blood.2023020509. Blood. 2023. PMID: 37383006 No abstract available.
References
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
-
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
-
- McMullin MF, Wilkins BS, Harrison CN. Management of polycythaemia vera: a critical review of current data. Br J Haematol. 2016;172(3):337–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

