Novel mouse models based on intersectional genetics to identify and characterize plasmacytoid dendritic cells
- PMID: 36928414
- PMCID: PMC10063451
- DOI: 10.1038/s41590-023-01454-9
Novel mouse models based on intersectional genetics to identify and characterize plasmacytoid dendritic cells
Abstract
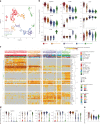
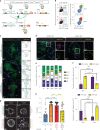
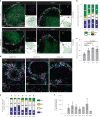
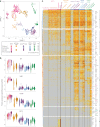
Plasmacytoid dendritic cells (pDCs) are the main source of type I interferon (IFN-I) during viral infections. Their other functions are debated, due to a lack of tools to identify and target them in vivo without affecting pDC-like cells and transitional DCs (tDCs), which harbor overlapping phenotypes and transcriptomes but a higher efficacy for T cell activation. In the present report, we present a reporter mouse, pDC-Tom, designed through intersectional genetics based on unique Siglech and Pacsin1 coexpression in pDCs. The pDC-Tom mice specifically tagged pDCs and, on breeding with Zbtb46GFP mice, enabled transcriptomic profiling of all splenic DC types, unraveling diverging activation of pDC-like cells versus tDCs during a viral infection. The pDC-Tom mice also revealed initially similar but later divergent microanatomical relocation of splenic IFN+ versus IFN- pDCs during infection. The mouse models and specific gene modules we report here will be useful to delineate the physiological functions of pDCs versus other DC types.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













Comment in
-
Plasmacytoid dendritic cells turn red!Nat Immunol. 2023 Apr;24(4):563-564. doi: 10.1038/s41590-023-01472-7. Nat Immunol. 2023. PMID: 36959295 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

