Pembrolizumab Added to Ifosfamide, Carboplatin, and Etoposide Chemotherapy for Relapsed or Refractory Classic Hodgkin Lymphoma: A Multi-institutional Phase 2 Investigator-Initiated Nonrandomized Clinical Trial
- PMID: 36928527
- PMCID: PMC10020934
- DOI: 10.1001/jamaoncol.2022.7975
Pembrolizumab Added to Ifosfamide, Carboplatin, and Etoposide Chemotherapy for Relapsed or Refractory Classic Hodgkin Lymphoma: A Multi-institutional Phase 2 Investigator-Initiated Nonrandomized Clinical Trial
Abstract
Importance: To our knowledge, this is the first clinical trial designed to investigate concurrent treatment with a checkpoint inhibitor and conventional chemotherapy in relapsed or refractory classic Hodgkin lymphoma in patients destined for an autologous stem cell transplant.
Objective: To evaluate the complete response rate as assessed by 18F-fluorodeoxyglucose-positron emission tomography with computed tomography (FDG-PET/CT) after salvage therapy for patients with relapsed or refractory classic Hodgkin lymphoma.
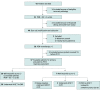
Design, setting, and participants: A single-group, phase 2, multi-institutional nonrandomized clinical trial to evaluate the addition of pembrolizumab to ifosfamide, carboplatin, and etoposide (ICE) chemotherapy was conducted from April 20, 2017, to October 29, 2020, at 5 US sites. The 42 patients were aged 18 years or older, with an Eastern Cooperative Oncology Group Performance Status Scale score of 0 or 1 and biopsy-proven relapsed or refractory classic Hodgkin lymphoma after 1 or 2 prior lines of chemotherapy. Patients were required to be appropriate candidates for transplant, with measurable lesions detected by FDG-PET/CT.
Interventions: Two cycles of pembrolizumab (200 mg intravenously on day 1) with ICE chemotherapy every 21 days, followed by stem cell mobilization and collection, and then 1 cycle of pembrolizumab monotherapy followed by FDG-PET/CT response assessment.
Main outcomes and measures: The primary end point was complete response rate detected by FDG-PET/CT, defined as a Deauville score of 3 or lower. Patients with a complete response proceeded to an autologous stem cell transplant. Secondary end points included progression-free survival, overall survival, stem cell mobilization, and neutrophil and platelet engraftment. Adverse events were monitored to assess safety.
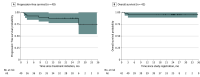
Results: Forty-two patients were enrolled, with 37 evaluable for the primary end point. The median age was 34 years (range, 19-70 years), 25 patients were female (68%), 6 were African American (16%), and 26 were White (70%). The complete response rate for the 37 patients assessed by FDG-PET/CT imaging was 86.5% (95% CI, 71.2%-95.5%); the overall response rate was 97.3% (36 patients), with 10.8% partial responses (4 patients). New areas of FDG-PET positivity in 2 patients were biopsied, showing noncaseating granuloma in 1 case and a reactive lymph node in a second. Progression-free survival and overall survival 2-year estimates were 87.2% (32 patients; 95% CI, 77.3%-98.3%) and 95.1% (95% CI, 88.8%-100%), respectively. The addition of pembrolizumab to ICE chemotherapy did not negatively affect stem cell mobilization or collection or engraftment, similar to prior experience in this patient population and setting.
Conclusions and relevance: Results suggest that the addition of pembrolizumab to ICE chemotherapy was well tolerated and highly effective in comparison with prior reports of chemotherapy-only regimens, supporting further investigation in patients with relapsed or refractory classic Hodgkin lymphoma eligible for an autologous stem cell transplant.
Trial registration: ClinicalTrials.gov Identifier: NCT03077828.
Conflict of interest statement
Figures
References
-
- Gordon LI, Hong F, Fisher RI, et al. . Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684-691. doi:10.1200/JCO.2012.43.4803 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

