Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice
- PMID: 36928635
- PMCID: PMC10208560
- DOI: 10.1038/s41593-023-01271-1
Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice
Abstract
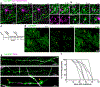
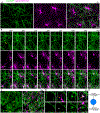
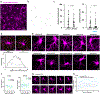
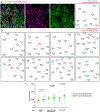
Myelin degeneration occurs in neurodegenerative diseases and aging. In these conditions, resident oligodendrocyte progenitor cells (OPCs) differentiate into oligodendrocytes that carry out myelin repair. To investigate the cellular dynamics underlying these events, we developed a noninflammatory demyelination model that combines intravital two-photon imaging with a single-cell ablation technique called two-photon apoptotic targeted ablation (2Phatal). Oligodendrocyte 2Phatal in both sexes results in a myelin degeneration cascade that triggers rapid forms of synchronous remyelination on defined axons. This remyelination is driven by oligodendrocytes differentiated from a subset of morphologically distinct, highly branched OPCs. Moreover, remyelination efficiency depends on the initial myelin patterns, as well as the age of the organism. In summary, using 2Phatal, we show a form of rapid synchronous remyelination, mediated by a distinct subset of OPCs, capable of restoring the original myelin patterning in adulthood but not aging.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures











References
-
- Zalc B, Goujet D & Colman D The origin of the myelination program in vertebrates. Curr. Biol 18, R511–2 (2008). - PubMed
-
- Stadelmann C, Timmler S, Barrantes-Freer A & Simons M Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev 99, 1381–1431 (2019). - PubMed
-
- Franklin RJM & Ffrench-Constant C Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci 9, 839–855 (2008). - PubMed
-
- Nave K-A Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

