KIF13B mediates VEGFR2 recycling to modulate vascular permeability
- PMID: 36928770
- PMCID: PMC10165967
- DOI: 10.1007/s00018-023-04752-5
KIF13B mediates VEGFR2 recycling to modulate vascular permeability
Abstract
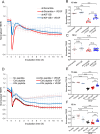
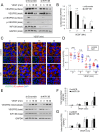
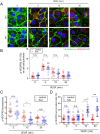
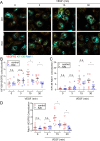
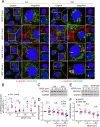
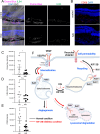
Excessive vascular endothelial growth factor-A (VEGF-A) signaling induces vascular leakage and angiogenesis in diseases. VEGFR2 trafficking to the cell surface, mediated by kinesin-3 family protein KIF13B, is essential to respond to VEGF-A when inducing angiogenesis. However, the precise mechanism of how KIF13B regulates VEGF-induced signaling and its effects on endothelial permeability is largely unknown. Here we show that KIF13B-mediated recycling of internalized VEGFR2 through Rab11-positive recycling vesicle regulates endothelial permeability. Phosphorylated VEGFR2 at the cell-cell junction was internalized and associated with KIF13B in Rab5-positive early endosomes. KIF13B mediated VEGFR2 recycling through Rab11-positive recycling vesicle. Inhibition of the function of KIF13B attenuated phosphorylation of VEGFR2 at Y951, SRC at Y416, and VE-cadherin at Y685, which are necessary for endothelial permeability. Failure of VEGFR2 trafficking to the cell surface induced accumulation and degradation of VEGFR2 in lysosomes. Furthermore, in the animal model of the blinding eye disease wet age-related macular degeneration (AMD), inhibition of KIF13B-mediated VEGFR2 trafficking also mitigated vascular leakage. Thus, the present results identify the fundamental role of VEGFR2 recycling to the cell surface in mediating vascular permeability, which suggests a promising strategy for mitigating vascular leakage associated with inflammatory diseases.
Keywords: Endothelial permeability; Eye diseases; Kinesin; Trafficking; VEGFR2; Vesicle.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Authors have no conflict of interests that might be perceived to influence the results and/or discussion reported in this article.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

