Co-administration of AYUSH 64 as an adjunct to standard of care in mild and moderate COVID-19: A randomized, controlled, multicentric clinical trial
- PMID: 36928877
- PMCID: PMC10019690
- DOI: 10.1371/journal.pone.0282688
Co-administration of AYUSH 64 as an adjunct to standard of care in mild and moderate COVID-19: A randomized, controlled, multicentric clinical trial
Abstract
Objective: Evaluate the efficacy of AYUSH 64, a standard polyherbal Ayurvedic drug in COVID-19.
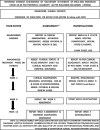
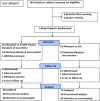
Methods: During the first pandemic wave, 140 consenting and eligible hospitalized adult participants with mild-moderate symptomatic disease (specific standard RT-PCR assay positive) were selected as per a convenience sample, and randomized (1:1 ratio) to an open-label (assessor blind) two-arm multicentric drug trial; standard of care (SOC as per Indian guidelines) versus AYUSH 64 combined with SOC (AYUSH plus). Participants were assessed daily and discharged once clinical recovery (CR, primary efficacy) was achieved which was based on a predetermined set of criteria (resolution of symptoms, normal peripheral oximetry, and negative specific RT-PCR assay). Each participant was followed using an indigenous software program(mobile phone) and completed a 12-week study period. The dose of AYUSH 64 was 2 tablets oral, 500 mg each, bid for 12 weeks (AYUSH plus only). Significant P was <0.05 (two-sided). On randomization, the groups were found well matched.
Results: The mean interval time from randomization to CR was significantly superior in the AYUSH plus group [mean 6.45 days versus 8.26 days, 95% Confidence Interval of the difference -3.02 to -0.59 (P = 0.003, Student's 't test] as per-protocol analysis (134 participants); significant (P = 0.002) on an intention to treat analysis. 70% of the participants in AYUSH plus recovered during the first week (P = 0.046, Chi-square) and showed a significantly better change in physical health, fatigue, and quality of life measures. 48 adverse events, mostly mild and gut related, were reported by each group. There were 20 patient withdrawals (8 in AYUSH plus) but none due to an AE. There were no deaths. Daily assessment (hospitalization) and supervised drug intake ensured robust efficacy data. The open-label design was a concern (study outcome).
Conclusions: AYUSH 64 in combination with SOC hastened recovery, reduced hospitalization, and improved health in COVID-19. It was considered safe and well-tolerated. Further clinical validation (Phase III) is required.
Trial registration: CTRI/2020/06/025557.
Copyright: © 2023 Chopra et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist. The authors declare their relationship related to study as per the International Committee of Medical Journal Editors is described in the section on ‘Financial Disclosure’ (see above). "This does not alter our adherence to PLOS ONE policies on sharing data and materials.”
Figures
References
-
- Khheirabadi D, Haddad F, Mousavi-Roknabadi RS, Rezaeisadrabadi M, Dehghan H, Fazlzadeh A. A complementary critical appraisal on systematic reviews regarding the most efficient therapeutic strategies for the current COVID-19 (SARS-CoV-2) pandemic. J Med Virol. 2021;93: 2705–2721. doi: 10.1002/jmv.26811 - DOI - PMC - PubMed
-
- NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. NIH. 2020. Available from: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treat... - PubMed
-
- Guidelines on Clinical Management of COVID-19 issued by Ministry of Health and Family Planning, Government of India (17 Mar 2020). Available from https://www.mohfw.gov.in/pdf/GuidelinesonClinicalManagementofCOVID191202...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical