Efficacy of platelet-inspired hemostatic nanoparticles on bleeding in von Willebrand disease murine models
- PMID: 36928925
- PMCID: PMC10315625
- DOI: 10.1182/blood.2022018956
Efficacy of platelet-inspired hemostatic nanoparticles on bleeding in von Willebrand disease murine models
Abstract
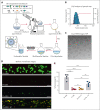
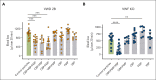
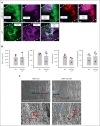
The lack of innovation in von Willebrand disease (VWD) originates from many factors including the complexity and heterogeneity of the disease but also from a lack of recognition of the impact of the bleeding symptoms experienced by patients with VWD. Recently, a few research initiatives aiming to move past replacement therapies using plasma-derived or recombinant von Willebrand factor (VWF) concentrates have started to emerge. Here, we report an original approach using synthetic platelet (SP) nanoparticles for the treatment of VWD type 2B (VWD-2B) and severe VWD (type 3 VWD). SP are liposomal nanoparticles decorated with peptides enabling them to concomitantly bind to collagen, VWF, and activated platelets. In vitro, using various microfluidic assays, we show the efficacy of SPs to improve thrombus formation in VWF-deficient condition (with human platelets) or using blood from mice with VWD-2B and deficient VWF (VWF-KO, ie, type 3 VWD). In vivo, using a tail-clip assay, SP treatment reduced blood loss by 35% in mice with VWD-2B and 68% in mice with VWF-KO. Additional studies using nanoparticles decorated with various combinations of peptides demonstrated that the collagen-binding peptide, although not sufficient by itself, was crucial for SP efficacy in VWD-2B; whereas all 3 peptides appeared necessary for mice with VWF-KO. Clot imaging by immunofluorescence and scanning electron microscopy revealed that SP treatment of mice with VWF-KO led to a strong clot, similar to those obtained in wild-type mice. Altogether, our results show that SP could represent an attractive therapeutic alternative for VWD, especially considering their long half-life and stability.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.D., E.G., C.P., and M.B. are employees of Haima Therapeutics LLC. A.S.G. is cofounder and chief scientific adviser for Haima Therapeutics. The remaining authors declare no competing financial interests.
Figures



References
-
- Denis CV, Susen S, Lenting PJ. von Willebrand disease: what does the future hold? Blood. 2021;137(17):2299–2306. - PubMed
-
- Federici AB, Mannucci PM, Castaman G, et al. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: a cohort study of 67 patients. Blood. 2009;113(3):526–534. - PubMed
-
- Kruse-Jarres R, Johnsen JM. How I treat type 2B von Willebrand disease. Blood. 2018;131(12):1292–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

