Population characteristics, management, and survival outcomes in muscle-invasive urothelial carcinoma undergoing radical resection: the MINOTAUR study
- PMID: 36929410
- PMCID: PMC10018592
- DOI: 10.1007/s00345-023-04335-w
Population characteristics, management, and survival outcomes in muscle-invasive urothelial carcinoma undergoing radical resection: the MINOTAUR study
Abstract
Purpose: To describe the incidence, management, and survival outcomes of patients with muscle-invasive urothelial carcinoma (MIUC) undergoing radical surgery (RS) in France.
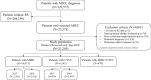
Methods: We relied on a non-interventional real-world retrospective study based on French National Hospitalization Database. Adults with MIUC with a first RS between 2015 and 2020 were selected. Subpopulations of patients with RS performed in 2015 and 2019 (pre-COVID-19) were extracted, according to cancer site: muscle-invasive bladder cancer (MIBC) or upper tract urothelial carcinoma (UTUC). Disease-free and overall survival (DFS, OS - Kaplan-Meier) were assessed on the 2015 subpopulation.
Results: Between 2015 and 2020, 21,295 MIUC patients underwent a first RS. Of them, 68.9% had MIBC, 28.9% UTUC, and 2.2% both cancers. Apart from fewer men among UTUC (70.2%) than MIBC patients (90.1%), patients' demographic (mean age ~ 73 years) and clinical characteristics were similar whatever the cancer site or year of first RS. In 2019, RS alone was the most frequent treatment, occurring in 72.3% and 92.6% in MIBC and UTUC, respectively. Between 2015 and 2019, neoadjuvant use rate increased from 13.8% to 22.2% in MIBC, and adjuvant use rate increased from 3.7% to 6.3% in UTUC. Finally, median [95% confidence interval] DFS times were 16.0 [14.0-18.0] and 27.0 [23.0-32.0] months among MIBC and UTUC, respectively.
Conclusion: Among patients with resected MIUC annually, RS alone remained the main treatment. Neoadjuvant and adjuvant use increased between 2015 and 2019. Nonetheless, MIUC remains of poor prognosis, highlighting an unmet medical need, notably among patients at high risk of recurrence.
Keywords: (neo)Adjuvant chemotherapy; Bladder cancer; Insurance claim review; Renal pelvis; Survival outcomes; Ureter; Urothelial carcinoma.
© 2023. The Author(s).
Conflict of interest statement
MR received personal fees from Astra Zeneca, Astellas, Bristol Myers Squibb, Janssen and Roche. FC, AP, MC, AFG and SBr are employees of Bristol Myers Squibb. AB, PDI, FB and LC are employees of stève consultants, which has a research consultancy contract with Bristol Myers Squibb. SBe is the executive director of stève consultants, which has a research consultancy contract with Bristol Myers Squibb. CB received personal fees from Bristol Myers Squibb. SN received consulting fees from Bristol Myers Squibb, Ipsen, Pfizer, Merck Sharpe & Dome and Eisai.
Figures

References
-
- Defossez G, Le Guyader-Peyrou S, Uhry Z, Grosclaude P, Remontet L, Colonna M. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Étude à partir des registres des cancers du réseau Francim. Volume 1 - Tumeurs solides. Compléments par localisation tumorale et sous-sites ou sous-types histologiques. Saint-Maurice: Santé Publique France; 2019.
-
- Rouprêt M, Audenet F, Roumiguié M, Pignot G, Masson-Lecomte A, Compérat E, et al. Recommandations françaises du Comité de cancérologie de l’AFU - actualisation 2020–2022 : tumeurs de la voie excrétrice urinaire supérieure. Progrès en Urologie. 2020;30(12, Supplement):S52–77. - PubMed
-
- Rouprêt M, Pignot G, Masson-Lecomte A, Compérat E, Audenet F, Roumiguié M, et al. Recommandations françaises du Comité de cancérologie de l’AFU – actualisation 2020–2022 : tumeurs de la vessie. Progrès en Urologie. 2020;30(12, Supplement):S78–135. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

