Significant impact of time-of-day variation on metformin pharmacokinetics
- PMID: 36930251
- PMCID: PMC10163090
- DOI: 10.1007/s00125-023-05898-4
Significant impact of time-of-day variation on metformin pharmacokinetics
Abstract
Aims/hypothesis: The objective was to investigate if metformin pharmacokinetics is modulated by time-of-day in humans using empirical and mechanistic pharmacokinetic modelling techniques on a large clinical dataset. This study also aimed to generate and test hypotheses on the underlying mechanisms, including evidence for chronotype-dependent interindividual differences in metformin plasma and efficacy-related tissue concentrations.
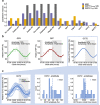
Methods: A large clinical dataset consisting of individual metformin plasma and urine measurements was analysed using a newly developed empirical pharmacokinetic model. Causes of daily variation of metformin pharmacokinetics and interindividual variability were further investigated by a literature-informed mechanistic modelling analysis.
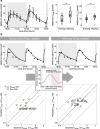
Results: A significant effect of time-of-day on metformin pharmacokinetics was found. Daily rhythms of gastrointestinal, hepatic and renal processes are described in the literature, possibly affecting drug pharmacokinetics. Observed metformin plasma levels were best described by a combination of a rhythm in GFR, renal plasma flow (RPF) and organic cation transporter (OCT) 2 activity. Furthermore, the large interindividual differences in measured metformin concentrations were best explained by individual chronotypes affecting metformin clearance, with impact on plasma and tissue concentrations that may have implications for metformin efficacy.
Conclusions/interpretation: Metformin's pharmacology significantly depends on time-of-day in humans, determined with the help of empirical and mechanistic pharmacokinetic modelling, and rhythmic GFR, RPF and OCT2 were found to govern intraday variation. Interindividual variation was found to be partly dependent on individual chronotype, suggesting diurnal preference as an interesting, but so-far underappreciated, topic with regard to future personalised chronomodulated therapy in people with type 2 diabetes.
Keywords: Chronopharmacology; Empirical modelling; Mechanistic modelling; Metformin; Pharmacokinetics; Renal excretion; Transporter.
© 2023. The Author(s).
Figures


Comment in
-
Variation in metformin pharmacokinetics.Nat Rev Endocrinol. 2023 Jun;19(6):314. doi: 10.1038/s41574-023-00834-3. Nat Rev Endocrinol. 2023. PMID: 37012422 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

