An evolutionarily conserved pacemaker role for HCN ion channels in smooth muscle
- PMID: 36930567
- PMCID: PMC10065941
- DOI: 10.1113/JP283701
An evolutionarily conserved pacemaker role for HCN ion channels in smooth muscle
Abstract
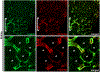
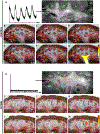
Although hyperpolarization-activated cation (HCN) ion channels are well established to underlie cardiac pacemaker activity, their role in smooth muscle organs remains controversial. HCN-expressing cells are localized to renal pelvic smooth muscle (RPSM) pacemaker tissues of the murine upper urinary tract and HCN channel conductance is required for peristalsis. To date, however, the Ih pacemaker current conducted by HCN channels has never been detected in these cells, raising questions on the identity of RPSM pacemakers. Indeed, the RPSM pacemaker mechanisms of the unique multicalyceal upper urinary tract exhibited by humans remains unknown. Here, we developed immunopanning purification protocols and demonstrate that 96% of isolated HCN+ cells exhibit Ih . Single-molecule STORM to whole-tissue imaging showed HCN+ cells express single HCN channels on their plasma membrane and integrate into the muscular syncytium. By contrast, PDGFR-α+ cells exhibiting the morphology of ICC gut pacemakers were shown to be vascular mural cells. Translational studies in the homologous human and porcine multicalyceal upper urinary tracts showed that contractions and pacemaker depolarizations originate in proximal calyceal RPSM. Critically, HCN+ cells were shown to integrate into calyceal RPSM pacemaker tissues, and HCN channel block abolished electrical pacemaker activity and peristalsis of the multicalyceal upper urinary tract. Cumulatively, these studies demonstrate that HCN ion channels play a broad, evolutionarily conserved pacemaker role in both cardiac and smooth muscle organs and have implications for channelopathies as putative aetiologies of smooth muscle disorders. KEY POINTS: Pacemakers trigger contractions of involuntary muscles. Hyperpolarization-activated cation (HCN) ion channels underpin cardiac pacemaker activity, but their role in smooth muscle organs remains controversial. Renal pelvic smooth muscle (RPSM) pacemakers trigger contractions that propel waste away from the kidney. HCN+ cells localize to murine RPSM pacemaker tissue and HCN channel conductance is required for peristalsis. The HCN (Ih ) current has never been detected in RPSM cells, raising doubt whether HCN+ cells are bona fide pacemakers. Moreover, the pacemaker mechanisms of the unique multicalyceal RPSM of higher order mammals remains unknown. In total, 97% of purified HCN+ RPSM cells exhibit Ih . HCN+ cells integrate into the RPSM musculature, and pacemaker tissue peristalsis is dependent on HCN channels. Translational studies in human and swine demonstrate HCN channels are conserved in the multicalyceal RPSM and that HCN channels underlie pacemaker activity that drives peristalsis. These studies provide insight into putative channelopathies that can underlie smooth muscle dysfunction.
Keywords: HCN channels; Ih funny current; PDGFR-α; pacemakers; peristalsis; renal pelvis; smooth muscle.
© 2023 The Authors. The Journal of Physiology © 2023 The Physiological Society.
Figures








References
-
- Barres BA. (2014). Designing and troubleshooting immunopanning protocols for purifying neural cells. Cold Spring Harbor protocols 2014, 1342–1347. - PubMed
-
- Bassols A, Costa C, Eckersall PD, Osada J, Sabria J & Tibau J. (2014). The pig as an animal model for human pathologies: A proteomics perspective. Proteomics Clinical applications 8, 715–731. - PubMed
-
- Benzoni P, Bertoli G, Giannetti F, Piantoni C, Milanesi R, Pecchiari M, Barbuti A, Baruscotti M & Bucchi A. (2021). The funny current: Even funnier than 40 years ago. Uncanonical expression and roles of HCN/f channels all over the body. Prog Biophys Mol Biol. - PubMed
-
- Biel M, Wahl-Schott C, Michalakis S & Zong X. (2009). Hyperpolarization-activated cation channels: from genes to function. Physiological reviews 89, 847–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

