Transcriptomic Profiling Reveals Chemokine CXCL1 as a Mediator for Neutrophil Recruitment Associated With Blood-Retinal Barrier Alteration in Diabetic Retinopathy
- PMID: 36930735
- PMCID: PMC10202768
- DOI: 10.2337/db22-0619
Transcriptomic Profiling Reveals Chemokine CXCL1 as a Mediator for Neutrophil Recruitment Associated With Blood-Retinal Barrier Alteration in Diabetic Retinopathy
Abstract
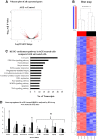
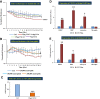
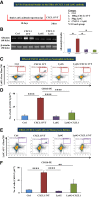
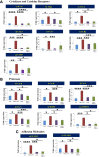
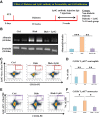
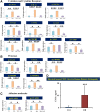
Inflammation plays an important role in the pathogenesis of diabetic retinopathy (DR). To precisely define the inflammatory mediators, we examined the transcriptomic profile of human retinal endothelial cells exposed to advanced glycation end products, which revealed the neutrophil chemoattractant chemokine CXCL1 as one of the top genes upregulated. The effect of neutrophils in the alteration of the blood-retinal barrier (BRB) was further assessed in wild-type C57BL/6J mice intravitreally injected with recombinant CXCL1 as well as in streptozotocin-induced diabetic mice. Both intravitreally CXCL1-injected and diabetic animals showed significantly increased retinal vascular permeability, with significant increase in infiltration of neutrophils and monocytes in retinas and increased expression of chemokines and their receptors, proteases, and adhesion molecules. Treatment with Ly6G antibody for neutrophil depletion in both diabetic mice as well as CXCL1-injected animals showed significantly decreased retinal vascular permeability accompanied by decreased infiltration of neutrophils and monocytes and decreased expression of cytokines and proteases. CXCL1 level was significantly increased in the serum samples of patients with DR compared with samples of those without diabetes. These data reveal a novel mechanism by which the chemokine CXCL1, through neutrophil recruitment, alters the BRB in DR and, thus, serves as a potential novel therapeutic target.
Article highlights: Intravitreal CXCL1 injection and diabetes result in increased retinal vascular permeability with neutrophil and monocyte recruitment. Ly6G antibody treatment for neutrophil depletion in both animal models showed decreased retinal permeability and decreased cytokine expression. CXCL1 is produced by retinal endothelial cells, pericytes, and astrocytes. CXCL1 level is significantly increased in serum samples of patients with diabetic retinopathy. CXCL1, through neutrophil recruitment, alters the blood-retinal barrier in diabetic retinopathy and, thus, may be used as a therapeutic target.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 2015;122:1375–1394 - PubMed
-
- Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 2007;56:2380–2387 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

