Dense and Acidic Organelle-Targeted Visualization in Living Cells: Application of Viscosity-Responsive Fluorescence Utilizing Restricted Access to Minimum Energy Conical Intersection
- PMID: 36930819
- PMCID: PMC10061370
- DOI: 10.1021/acs.analchem.2c04133
Dense and Acidic Organelle-Targeted Visualization in Living Cells: Application of Viscosity-Responsive Fluorescence Utilizing Restricted Access to Minimum Energy Conical Intersection
Abstract
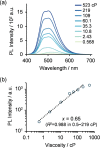
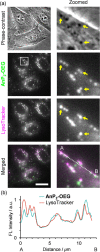
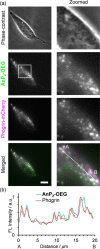
Cell-imaging methods with functional fluorescent probes are an indispensable technique to evaluate physical parameters in cellular microenvironments. In particular, molecular rotors, which take advantage of the twisted intramolecular charge transfer (TICT) process, have helped evaluate microviscosity. However, the involvement of charge-separated species in the fluorescence process potentially limits the quantitative evaluation of viscosity. Herein, we developed viscosity-responsive fluorescent probes for cell imaging that are not dependent on the TICT process. We synthesized AnP2-H and AnP2-OEG, both of which contain 9,10-di(piperazinyl)anthracene, based on 9,10-bis(N,N-dialkylamino)anthracene that adopts a nonflat geometry at minimum energy conical intersection. AnP2-H and AnP2-OEG exhibited enhanced fluorescence as the viscosity increased, with sensitivities comparable to those of conventional molecular rotors. In living cell systems, AnP2-OEG showed low cytotoxicity and, reflecting its viscosity-responsive property, allowed specific visualization of dense and acidic organelles such as lysosomes, secretory granules, and melanosomes under washout-free conditions. These results provide a new direction for developing functional fluorescent probes targeting dense organelles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

