Immediate treatment for recent hepatitis C infection in people with high-risk behaviors: a systematic review and meta-analysis
- PMID: 36930865
- PMCID: PMC10027039
- DOI: 10.1097/HC9.0000000000000082
Immediate treatment for recent hepatitis C infection in people with high-risk behaviors: a systematic review and meta-analysis
Abstract
Background and aims: Direct-acting antivirals (DAAs) are almost exclusively approved for the treatment of chronic HCV. This poses a significant barrier to the treatment of recently acquired HCV because of the limited access to DAAs. This review seeks to address this issue by synthesizing evidence of the benefits and harms of immediate treatment after the detection of recently acquired HCV in people at higher risk of infection.
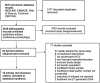
Approach and results: A systematic review and meta-analysis were conducted reporting on populations with recently acquired HCV at higher risk of infection. Studies were included if they assessed standard duration DAA treatment regimens and reported on the benefits and harms of immediate treatment (within one year of diagnosis). Outcomes included sustained virological response at 12 weeks post-treatment (SVR12), incidence, treatment initiation and adherence, overtreatment, engagement in care, and adverse events. Eight cohort studies, 3 open-label trials, and 1 case series study were included, reporting on 2085 participants with recently acquired HCV infection. No studies included a comparison group. Eight studies assessed DAA treatment in either men who have sex with men or men who have sex with men with HIV, 2 studies assessed treatment in people who inject drugs, and 2 among people living with HIV. Immediate treatment of HCV was associated with a pooled SVR12 of 95.9% (95% CI, 92.6%-99.3%). Three studies reported on hepatitis C incidence, where most participants were treated in the chronic phase of infection. A treatment completion rate of 100% was reported in 2 studies, and only 1 serious adverse event was described.
Conclusions: High rates of cure were achieved with the treatment of recently acquired hepatitis C in people at higher risk of infection. Serious adverse events were rare, highlighting individual benefits consistent with the treatment of chronic hepatitis C. The impact of immediate treatment on HCV incidence requires further evaluation.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Gail V. Matthews consults, advises, and received grants from Gilead Sciences. She consults and received grants from ViiV; she received grants from Abbvie. Joseph S. Doyle received grants from Gilead and Abbvie. Michael W. Traeger received grants from Gilead Science. The remaining authors have no conflicts to report.
Figures

Comment in
-
Kürzlich erworbene HCV-Infektion sofort behandeln!MMW Fortschr Med. 2023 Apr;165(8):11. doi: 10.1007/s15006-023-2598-4. MMW Fortschr Med. 2023. PMID: 37081330 German. No abstract available.
References
-
- World Health Organisation. Global Health Sector Strategy on Viral Hepatitis 2016-2021 Towards Ending Viral Hepatitis. World Health Organization; 2016. 2016.
-
- Jin F, Dore GJ, Matthews G, Luhmann N, Macdonald V, Bajis S, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:39–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

