Heat shock proteins and cancer: The FoxM1 connection
- PMID: 36931349
- PMCID: PMC10134075
- DOI: 10.1016/j.bcp.2023.115505
Heat shock proteins and cancer: The FoxM1 connection
Abstract
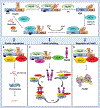
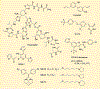
Heat shock proteins (Hsp) and FoxM1 have significant roles in carcinogenesis. According to their relative molecular weight, Hsps are divided into Hsp110, Hsp90, Hsp70, Hsp60, Hsp40, and small Hsps. Hsp70 can play essential functions in cancer initiation and is overexpressed in several human cancers. Hsp70, in combination with cochaperones HIP and HOP, refolds partially denatured proteins and acts as a cochaperone for Hsp90. Also, Hsp70, in combination with BAG3, regulates the FoxM1 signaling pathway. FoxM1 protein is a transcription factor of the Forkhead family that is overexpressed in most human cancers and is involved in many cancers' development features, including proliferation, migration, invasion, angiogenesis, metastasis, and resistance to apoptosis. This review discusses the Hsp70, Hsp90, and FoxM1 structure and function, the known Hsp70 cochaperones, and Hsp70, Hsp90, and FoxM1 inhibitors.
Keywords: Cancer; Cochaperones; FoxM1; Hsp70; Hsp90; Inhibitors.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


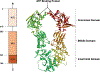


References
-
- Parcellier A; Gurbuxani S; Schmitt E; Solary E; Garrido C, Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochemical and Biophysical Research Communications 2003, 304, (3), 505–512. - PubMed
-
- Seneci P, Chapter 6 - Assembly and Disassembly of Protein Aggregates: Unraveling the Maze. In Molecular Targets in Protein Misfolding and Neurodegenerative Disease, Seneci P, Ed. Press Academic: San Diego, 2015; pp 229–276.
-
- Kozeko LY, The Role of HSP90 Chaperones in Stability and Plasticity of Ontogenesis of Plants under Normal and Stressful Conditions (Arabidopsis thaliana). Cytology and Genetics 2019, 53, (2), 143–161.
-
- Ran R; Lu A; Xu H; Tang Y; Sharp FR, Heat-Shock Protein Regulation of Protein Folding, Protein Degradation, Protein Function, and Apoptosis. In Handbook of Neurochemistry and Molecular Neurobiology: Acute Ischemic Injury and Repair in the Nervous System, Lajtha A; Chan PH, Eds. Springer US: Boston, MA, 2007; pp 89–07.
-
- Mitra A; Shevde LA; Samant RS, Multi-faceted role of HSP40 in cancer. Clinical & Experimental Metastasis 2009, 26, (6), 559–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

