Initial Feasibility and Acute Toxicity Outcomes From a Phase 2 Trial of 18F-Fluorodeoxyglucose Positron Emission Tomography Response-Based De-escalated Definitive Chemoradiotherapy for p16+ Oropharynx Cancer: A Planned Interim Analysis
- PMID: 36931572
- PMCID: PMC12273592
- DOI: 10.1016/j.ijrobp.2023.03.043
Initial Feasibility and Acute Toxicity Outcomes From a Phase 2 Trial of 18F-Fluorodeoxyglucose Positron Emission Tomography Response-Based De-escalated Definitive Chemoradiotherapy for p16+ Oropharynx Cancer: A Planned Interim Analysis
Abstract
Purpose: 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) parameters are prognostic of oncologic outcomes in human papillomavirus-associated oropharyngeal squamous cell carcinoma (OPSCC). We used FDG-PET imaging biomarkers to select patients for de-escalated chemoradiotherapy (CRT), hypothesizing that acute toxicity will be improved with de-escalation.
Methods and materials: This is a planned interim initial feasibility and acute toxicity report from a phase 2, prospective, nonrandomized study, which enrolled patients with stage I-II p16+ OPSCC. All patients started definitive CRT to 70 Gy in 35 fractions, and those who met de-escalation criteria on midtreatment FDG-PET at fraction 10 completed treatment at 54 Gy in 27 fractions. We report the acute toxicity and patient-reported outcomes for 59 patients with a minimum follow-up of 3 months.
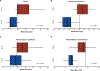
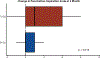
Results: There were no statistically significant differences between baseline patient characteristics in the standard and de-escalated cohorts. There were 28 of 59 (47.5%) patients who met FDG-PET de-escalation criteria and collectively received 20% to 30% less dose to critical organs at risk known to affect toxicity. At 3 months posttreatment, patients who received de-escalated CRT lost significantly less weight (median, 5.8% vs 13.0%; P < .001), had significantly less change from baseline in penetration-aspiration scale score (median, 0 vs 1; P = .018), and had significantly fewer aspiration events on repeat swallow study (8.0% vs 33.3%, P = .037) compared with patients receiving standard CRT.
Conclusions: Approximately half of patients with early-stage p16+ OPSCC are selected for de-escalation of definitive CRT using midtreatment FDG-PET biomarkers, which resulted in significantly improved rates of observed acute toxicity. Further follow-up is ongoing and will be required to determine whether this de-escalation approach preserves the favorable oncologic outcomes for patients with p16+ OPSCC before adoption.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures

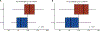
Similar articles
-
Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma.Cochrane Database Syst Rev. 2015 Jan 9;1(1):CD010533. doi: 10.1002/14651858.CD010533.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2025 Mar 26;3:CD010533. doi: 10.1002/14651858.CD010533.pub3. PMID: 25572491 Free PMC article. Updated.
-
Positron emission tomography-adapted therapy for first-line treatment in adults with Hodgkin lymphoma.Cochrane Database Syst Rev. 2025 Mar 26;3(3):CD010533. doi: 10.1002/14651858.CD010533.pub3. Cochrane Database Syst Rev. 2025. PMID: 40135712
-
Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma.Cochrane Database Syst Rev. 2016 Dec 11;12(12):CD010963. doi: 10.1002/14651858.CD010963.pub2. Cochrane Database Syst Rev. 2016. PMID: 27943254 Free PMC article.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
Interventions for the treatment of oral cavity and oropharyngeal cancers: surgical treatment.Cochrane Database Syst Rev. 2023 Aug 31;8(8):CD006205. doi: 10.1002/14651858.CD006205.pub5. Cochrane Database Syst Rev. 2023. PMID: 37650478 Free PMC article.
Cited by
-
Adaptive FDG-PET/CT guided dose escalation in head and neck squamous cell carcinoma: Late toxicity and oncologic outcomes (The ADMIRE study).Clin Transl Radiat Oncol. 2023 Sep 17;43:100676. doi: 10.1016/j.ctro.2023.100676. eCollection 2023 Nov. Clin Transl Radiat Oncol. 2023. PMID: 37753461 Free PMC article.
-
Adaptive radiotherapy for head and neck cancer: Pitfalls and possibilities from the radiation oncologist's point of view.Cancer Med. 2024 Apr;13(8):e7192. doi: 10.1002/cam4.7192. Cancer Med. 2024. PMID: 38650546 Free PMC article. Review.
-
Prospective Phase II Trial of Definitive Chemoradiation and Concurrent Nivolumab in Locally Advanced p16+ Oropharynx Cancer.Adv Radiat Oncol. 2025 Jul 10;10(10):101832. doi: 10.1016/j.adro.2025.101832. eCollection 2025 Oct. Adv Radiat Oncol. 2025. PMID: 40837213 Free PMC article.
References
-
- Marur S, Li S, Cmelak AJ, et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN cancer research group. J Clin Oncol 2017;35:490–497. - PMC - PubMed
-
- Seiwert TY, Foster CC, Blair EA, et al. Optima: A phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol 2019;30:297–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

