Colorectal polyps: Targets for fluorescence-guided endoscopy to detect high-grade dysplasia and T1 colorectal cancer
- PMID: 36931635
- PMCID: PMC10083466
- DOI: 10.1002/ueg2.12375
Colorectal polyps: Targets for fluorescence-guided endoscopy to detect high-grade dysplasia and T1 colorectal cancer
Abstract
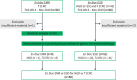
Background: Differentiating high-grade dysplasia (HGD) and T1 colorectal cancer (T1CRC) from low-grade dysplasia (LGD) in colorectal polyps can be challenging. Incorrect recognition of HGD or T1CRC foci can lead to a need for additional treatment after local resection, which might not have been necessary if it was recognized correctly. Tumor-targeted fluorescence-guided endoscopy might help to improve recognition.
Objective: Selecting the most suitable HGD and T1CRC-specific imaging target from a panel of well-established biomarkers: carcinoembryonic antigen (CEA), c-mesenchymal-epithelial transition factor (c-MET), epithelial cell adhesion molecule (EpCAM), folate receptor alpha (FRα), and integrin alpha-v beta-6 (αvβ6).
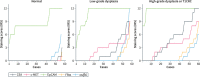
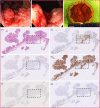
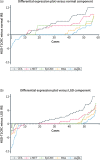
Methods: En bloc resection specimens of colorectal polyps harboring HGD or T1CRC were selected. Immunohistochemistry on paraffin sections was used to determine the biomarker expression in normal epithelium, LGD, HGD, and T1CRC (scores of 0-12). The differential expression in HGD-T1CRC components compared to surrounding LGD and normal components was assessed, just as the sensitivity and specificity of each marker.
Results: 60 specimens were included (21 HGD, 39 T1CRC). Positive expression (score >1) of HGD-T1CRC components was found in 73.3%, 78.3%, and 100% of cases for CEA, c-MET, and EpCAM, respectively, and in <40% for FRα and αvβ6. Negative expression (score 0-1) of the LGD component occurred more frequently for CEA (66.1%) than c-MET (31.6%) and EpCAM (0%). The differential expression in the HGD-T1CRC component compared to the surrounding LGD component was found for CEA in 66.7%, for c-MET in 43.1%, for EpCAM in 17.2%, for FRα in 22.4%, and for αvβ6 in 15.5% of the cases. Moreover, CEA showed the highest combined sensitivity (65.0%) and specificity (75.0%) for the detection of an HGD-T1CRC component in colorectal polyps.
Conclusion: Of the tested targets, CEA appears the most suitable to specifically detect HGD and T1 cancer foci in colorectal polyps. An in vivo study using tumor-targeted fluorescence-guided endoscopy should confirm these findings.
Keywords: CRC; advance; biomarkers; colorectal cancer; colorectal polyps; dysplasia; endoscopy; fluorescence; resection; target.
© 2023 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Jurjen Boonstra is a consultant at Boston Scientific.
Figures

References
-
- Backes Y, Schwartz MP, ter Borg F, Wolfhagen FHJ, Groen JN, de Vos tot Nederveen Cappel WH, et al. Multicentre prospective evaluation of real‐time optical diagnosis of T1 colorectal cancer in large non‐pedunculated colorectal polyps using narrow band imaging (the OPTICAL study). Gut. 2019;68(2):271–9, 10.1136/gutjnl-2017-314723 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

