Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance
- PMID: 36931693
- PMCID: PMC10314011
- DOI: 10.1136/ard-2022-223715
Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance
Erratum in
-
Correction: Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance.Ann Rheum Dis. 2024 Feb 15;83(3):e11. doi: 10.1136/ard-2022-223715corr1. Ann Rheum Dis. 2024. PMID: 38359929 Free PMC article. No abstract available.
Abstract
Objectives: Based on primary results from ORAL Surveillance, an event-driven clinical trial of risk-enriched patients, identify subpopulations with different relative risk (ie, 'high-risk' and 'low-risk') with tofacitinib versus tumour necrosis factor inhibitors (TNFi).
Methods: Patients with rheumatoid arthritis aged ≥50 years with ≥1 additional cardiovascular risk factor received tofacitinib 5 or 10 mg two times a day or TNFi. Prior analyses had identified age and smoking as risk factors of particular interest across safety outcomes. Hazard ratios (HRs) and incidence rates were evaluated by age and smoking individually and in combination. Results were validated across tofacitinib development programmes.
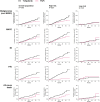
Results: 'Age ≥65 years or ever smoker' defined a group ('high-risk') with increased risk of malignancies (excluding non-melanoma skin cancer), major adverse cardiovascular events, myocardial infarction, venous thromboembolism and all-cause death with tofacitinib (combined doses) versus TNFi (HRs 1.41-5.19). In patients 'aged <65 years and never smokers' ('low-risk'), there was no detectable risk increase with tofacitinib versus TNFi (HRs ≈1.0) up to 6 years of follow-up, and absolute risk remained low and was corroborated across tofacitinib rheumatoid arthritis, psoriatic arthritis and ulcerative colitis programmes with up to 10 years of observation.
Conclusions: This posthoc analysis of ORAL Surveillance identified two tofacitinib subpopulations with different relative risk versus TNFi. High risk was confined to patients defined by distinct risk factors age ≥65 years or smoking, and these differentiating risk factors accounted for the excess risk observed with tofacitinib versus TNFi. These findings can guide individualised benefit/risk assessment and clinical decision-making on treatment with tofacitinib.
Trial registration numbers: NCT02092467, NCT01262118, NCT01484561, NCT00147498, NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT01164579, NCT00976599, NCT01059864, NCT01359150, NCT02147587, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385, NCT01039688, NCT02281552, NCT02187055, NCT02831855, NCT00413699, NCT00661661, NCT00787202, NCT01465763, NCT01458951, NCT01458574, NCT01470612, NCT01877668, NCT01882439, NCT01976364.
Keywords: Antirheumatic Agents; Arthritis; Therapeutics; Tumor Necrosis Factor Inhibitors.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LEK has received fees for speaking and consultancy from Pfizer, AbbVie, Amgen, Galapagos, UCB, Gilead, Biogen, BMS, MSD, Novartis, Eli Lilly and Janssen pharmaceuticals. LEK has received IIT research grants from Novo, UCB, Eli Lilly, Novartis and AbbVie. SD reports consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial, Vifor. SD reports lecture fees from AbbVie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, Takeda. AY, CW, EN, IM, JR and BB are employees and stockholders of Pfizer Inc.
Figures



References
-
- FDA . Approval letter - xeljanz (tofacitinib); 2012. Available: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203214Orig1s000A...
-
- Charles-Schoeman C, Buch MH, Dougados M, et al. . Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from oral surveillance. Ann Rheum Dis 2023;82:119–29. 10.1136/ard-2022-222259 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

