COVID-19 vaccine hesitancy among people living with HIV in a low-resource setting: A multi-center study of prevalence, correlates and reasons
- PMID: 36932032
- PMCID: PMC9946883
- DOI: 10.1016/j.vaccine.2023.02.056
COVID-19 vaccine hesitancy among people living with HIV in a low-resource setting: A multi-center study of prevalence, correlates and reasons
Abstract
Background: Hesitancy to COVID-19 vaccine may worsen the burden of COVID-19 among people living with HIV (PLHIV), who are at a higher risk of COVID-19-related hospitalization and death, compared to HIV non-infected individuals. Therefore, we evaluate the predictors and reasons for COVID-19 vaccine hesitancy among unvaccinated PLHIV in six antiretroviral therapy (ART) clinics across northern Nigeria.
Methodology: In this cross-sectional study, conducted between October 2021 and February 2022 in six hospitals across two geopolitical regions of Nigeria, we utilized interviewer-administered questionnaires to assess COVID-19 vaccine hesitancy among a convenience sample of 790 eligible adult PLHIV. Hesitancy was defined as answering 'no' or 'maybe' to a question asking participants their willingness to accept the COVID-19 vaccine. A multivariate logistic regression model was used to estimate the adjusted odds ratio (aOR) and 95% confidence interval (CI) of the factors associated with COVID-19 vaccine hesitancy among PLHIV.
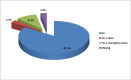
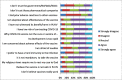
Results: Of the total 660 unvaccinated participants included in the analysis (61.82% female, mean age [SD] of 39.76 [10.75]), 381 (57.72%) were hesitant to COVID-19 vaccine. Being 50 years and older (aOR: 0.43; 95% CI: 0.21-0.89), being unemployed (aOR: 0.57; 95% CI: 0.34-0.95), experiencing the adverse effects of ART (aOR: 0.36; 95% CI: 0.15-0.86), and perception of being at high risk of contracting COVID-19 (aOR: 0.22; 95% CI: 0.13-0.37) were associated with significantly lower odds of hesitancy. Conversely, being female (aOR: 1.64; 95% CI: 1.02-2.61) and attending ART clinics at state administrative capital cities (IIDH Kano [aOR: 2.40; 95% CI: 1.10-5.25], MMSH Kano [aOR: 5.59; 95% CI: 1.97-10.66], YSSH Damaturu [aOR: 9.88; 95% CI: 4.02-24.29] vs. GH Gashua) were associated with significantly higher odds of hesitancy. The most common reasons for hesitancy include fear of potential adverse effects, skepticism about vaccine efficacy, the rapid development of the COVID-19 vaccine, and the perceived lack of effort to develop a cure or vaccine for HIV/AIDS.
Conclusion: Interventions aimed at combating misperceptions and misinformation regarding the COVID-19 vaccination program may reduce the prevalence of COVID-19 vaccine hesitancy among unvaccinated PLHIV.
Keywords: Acceptance/Hesitancy; COVID-19 vaccine; HIV; Nigeria; Uptake/Vaccination.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Predictors of COVID-19 Vaccine Acceptability among Patients Living with HIV in Northern Nigeria: A Mixed Methods Study.Curr HIV Res. 2022;20(1):82-90. doi: 10.2174/1570162X19666211217093223. Curr HIV Res. 2022. PMID: 34923948
-
Prevalence, determinants, and reasons for malaria vaccine hesitancy among caregivers of under-five children in Nigeria: Results from a nationwide cross-sectional survey.Vaccine. 2023 Feb 17;41(8):1503-1512. doi: 10.1016/j.vaccine.2023.01.060. Epub 2023 Jan 31. Vaccine. 2023. PMID: 36725434
-
Rates and determinants of COVID-19 vaccine uptake among people living with HIV in Federal Capital Territory, Nigeria.Trans R Soc Trop Med Hyg. 2025 Mar 7;119(3):210-220. doi: 10.1093/trstmh/trae094. Trans R Soc Trop Med Hyg. 2025. PMID: 39558837
-
COVID-19 vaccine hesitancy in Africa: a scoping review.Glob Health Res Policy. 2022 Jul 19;7(1):21. doi: 10.1186/s41256-022-00255-1. Glob Health Res Policy. 2022. PMID: 35850783 Free PMC article.
-
Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in Eastern China: A cross-sectional survey.J Integr Med. 2022 Jan;20(1):34-44. doi: 10.1016/j.joim.2021.10.004. Epub 2021 Oct 26. J Integr Med. 2022. PMID: 34774463 Free PMC article. Review.
Cited by
-
The impact of the COVID-19 pandemic on people living with HIV: a cross-sectional study in Caracas, Venezuela.BMC Infect Dis. 2024 Jan 15;24(1):87. doi: 10.1186/s12879-023-08967-6. BMC Infect Dis. 2024. PMID: 38225550 Free PMC article.
-
HIV stigma and other barriers to COVID-19 vaccine uptake among Georgian people living with HIV/AIDS: A mixed-methods study.PLOS Glob Public Health. 2024 Mar 28;4(3):e0003069. doi: 10.1371/journal.pgph.0003069. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 38547297 Free PMC article.
-
Differential immunogenicity in people living with HIV with varying CD4 levels after bivalent mRNA COVID-19 booster vaccination.PLoS One. 2025 Apr 29;20(4):e0317940. doi: 10.1371/journal.pone.0317940. eCollection 2025. PLoS One. 2025. PMID: 40299994 Free PMC article.
-
Determinants of COVID-19 Vaccine Uptake Among People Living with Human Immunodeficiency Virus.J Int Assoc Provid AIDS Care. 2025 Jan-Dec;24:23259582251328861. doi: 10.1177/23259582251328861. Epub 2025 Apr 1. J Int Assoc Provid AIDS Care. 2025. PMID: 40170389 Free PMC article.
-
Assessing SARS-CoV-2 vaccine hesitancy among the people living with and without HIV from May to September 2022 in Blantyre, Malawi.Hum Vaccin Immunother. 2023 Aug 1;19(2):2222052. doi: 10.1080/21645515.2023.2222052. Epub 2023 Jun 15. Hum Vaccin Immunother. 2023. PMID: 37318328 Free PMC article.
References
-
- Bertagnolio S., Thwin S.S., Silva R., et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV. 2022;9(7):e486–e495. doi: 10.1016/S2352-3018(22)00097-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

