Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system
- PMID: 36932093
- PMCID: PMC10023681
- DOI: 10.1038/s41467-023-37187-4
Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system
Abstract
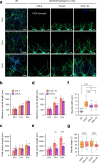
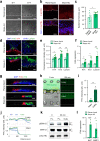
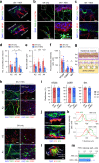
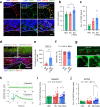
Reconstruction of skin equivalents with physiologically relevant cellular and matrix architecture is indispensable for basic research and industrial applications. As skin-nerve crosstalk is increasingly recognized as a major element of skin physiological pathology, the development of reliable in vitro models to evaluate the selective communication between epidermal keratinocytes and sensory neurons is being demanded. In this study, we present a three-dimensional innervated epidermal keratinocyte layer as a sensory neuron-epidermal keratinocyte co-culture model on a microfluidic chip using the slope-based air-liquid interfacing culture and spatial compartmentalization. Our co-culture model recapitulates a more organized basal-suprabasal stratification, enhanced barrier function, and physiologically relevant anatomical innervation and demonstrated the feasibility of in situ imaging and functional analysis in a cell-type-specific manner, thereby improving the structural and functional limitations of previous coculture models. This system has the potential as an improved surrogate model and platform for biomedical and pharmaceutical research.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
What about physical contacts between epidermal keratinocytes and sensory neurons?Exp Dermatol. 2018 Jan;27(1):9-13. doi: 10.1111/exd.13411. Epub 2017 Oct 19. Exp Dermatol. 2018. PMID: 28767170 Review.
-
Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model.J Invest Dermatol. 2013 Jun;133(6):1620-8. doi: 10.1038/jid.2012.464. Epub 2013 Jan 3. J Invest Dermatol. 2013. PMID: 23283070
-
Keratinocytes Communicate with Sensory Neurons via Synaptic-like Contacts.Ann Neurol. 2020 Dec;88(6):1205-1219. doi: 10.1002/ana.25912. Epub 2020 Oct 10. Ann Neurol. 2020. PMID: 32951274
-
Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model.Int J Mol Sci. 2020 May 29;21(11):3898. doi: 10.3390/ijms21113898. Int J Mol Sci. 2020. PMID: 32486109 Free PMC article.
-
Human Epidermal Keratinocytes in Culture: A Story of Multiple Recipes for a Single Cell Type.Skin Pharmacol Physiol. 2023;36(5):215-224. doi: 10.1159/000534137. Epub 2023 Sep 16. Skin Pharmacol Physiol. 2023. PMID: 37717566 Free PMC article. Review.
Cited by
-
Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications.Biomimetics (Basel). 2023 May 16;8(2):205. doi: 10.3390/biomimetics8020205. Biomimetics (Basel). 2023. PMID: 37218791 Free PMC article. Review.
-
Organoids/organs-on-chips towards biomimetic human artificial skin.Burns Trauma. 2025 May 3;13:tkaf029. doi: 10.1093/burnst/tkaf029. eCollection 2025. Burns Trauma. 2025. PMID: 40740685 Free PMC article. Review.
-
Characterization of AI-enhanced magnetophoretic transistors operating in a tri-axial magnetic field for on-chip bioparticle sorting.Sci Rep. 2024 Oct 8;14(1):23381. doi: 10.1038/s41598-024-74761-2. Sci Rep. 2024. PMID: 39379453 Free PMC article.
-
Fabrication and Validation of a 3D Portable PEGDA Microfluidic Chip for Visual Colorimetric Detection of Captured Breast Cancer Cells.Polymers (Basel). 2023 Jul 27;15(15):3183. doi: 10.3390/polym15153183. Polymers (Basel). 2023. PMID: 37571077 Free PMC article.
-
Advances in Microengineered Platforms for Skin Research.JID Innov. 2024 Sep 18;5(1):100315. doi: 10.1016/j.xjidi.2024.100315. eCollection 2025 Jan. JID Innov. 2024. PMID: 39525704 Free PMC article. Review.
References
-
- Boulais N, Misery L. The epidermis: a sensory tissue. Eur. J. Dermatol. 2008;18:119–127. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

