Telomere length associates with chronological age and mortality across racially diverse pulmonary fibrosis cohorts
- PMID: 36932145
- PMCID: PMC10023792
- DOI: 10.1038/s41467-023-37193-6
Telomere length associates with chronological age and mortality across racially diverse pulmonary fibrosis cohorts
Abstract
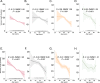
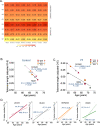
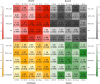
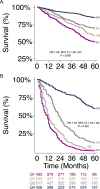
Pulmonary fibrosis (PF) is characterized by profound scarring and poor survival. We investigated the association of leukocyte telomere length (LTL) with chronological age and mortality across racially diverse PF cohorts. LTL measurements among participants with PF stratified by race/ethnicity were assessed in relation to age and all-cause mortality, and compared to controls. Generalized linear models were used to evaluate the age-LTL relationship, Cox proportional hazards models were used for hazard ratio estimation, and the Cochran-Armitage test was used to assess quartiles of LTL. Standardized LTL shortened with increasing chronological age; this association in controls was strengthened in PF (R = -0.28; P < 0.0001). In PF, age- and sex-adjusted LTL below the median consistently predicted worse mortality across all racial groups (White, HR = 2.21, 95% CI = 1.79-2.72; Black, HR = 2.22, 95% CI = 1.05-4.66; Hispanic, HR = 3.40, 95% CI = 1.88-6.14; and Asian, HR = 2.11, 95% CI = 0.55-8.23). LTL associates uniformly with chronological age and is a biomarker predictive of mortality in PF across racial groups.
© 2023. The Author(s).
Conflict of interest statement
A.A. declares speaking and advisory board fees from Genentech and Boehringer Ingelheim. C.A.N. declares consulting fees from Boehringer-Ingelheim. J.M.O. declares speaking and advisory board fees from Genentech and Boehringer Ingelheim. B.L. declares speaking fees from Genentech. P.J.W. declares personal fees from Blade therapeutics, Roche and Boehringer Ingelheim. F.J.M. declares personal fees from Copeval. I.N. declares honoraria for advisory boards with Boehringer Ingelheim, InterMune, and Anthera, speaking honoraria from GSK, and consulting fees for Immuneworks. M.E.S. declares honoraria from Genentech and editorial support from Boehringer Ingelheim. The remaining authors declare no competing interests.
Figures



Similar articles
-
Leukocyte Telomere Length and Risks of Incident Coronary Heart Disease and Mortality in a Racially Diverse Population of Postmenopausal Women.Arterioscler Thromb Vasc Biol. 2015 Oct;35(10):2225-31. doi: 10.1161/ATVBAHA.115.305838. Epub 2015 Aug 6. Arterioscler Thromb Vasc Biol. 2015. PMID: 26249011 Free PMC article.
-
Evaluation of Pulmonary Fibrosis Outcomes by Race and Ethnicity in US Adults.JAMA Netw Open. 2023 Mar 1;6(3):e232427. doi: 10.1001/jamanetworkopen.2023.2427. JAMA Netw Open. 2023. PMID: 36897590 Free PMC article.
-
Association of leukocyte telomere length with the risk of digestive diseases: A large-scale cohort study.Chin Med J (Engl). 2025 Jan 5;138(1):60-67. doi: 10.1097/CM9.0000000000002994. Epub 2024 Dec 6. Chin Med J (Engl). 2025. PMID: 39647990 Free PMC article.
-
Leukocyte telomere length and cardiovascular disease in African Americans: The Jackson Heart Study.Atherosclerosis. 2017 Nov;266:41-47. doi: 10.1016/j.atherosclerosis.2017.09.016. Epub 2017 Sep 15. Atherosclerosis. 2017. PMID: 28950166 Free PMC article.
-
Leukocyte Telomere Length and All-Cause, Cardiovascular Disease, and Cancer Mortality: Results From Individual-Participant-Data Meta-Analysis of 2 Large Prospective Cohort Studies.Am J Epidemiol. 2017 Jun 15;185(12):1317-1326. doi: 10.1093/aje/kww210. Am J Epidemiol. 2017. PMID: 28459963 Free PMC article.
Cited by
-
The role of autoantibodies in bridging obesity, aging, and immunosenescence.Immun Ageing. 2024 Nov 30;21(1):85. doi: 10.1186/s12979-024-00489-2. Immun Ageing. 2024. PMID: 39616399 Free PMC article. Review.
-
Role of Telomere Length in Survival of Patients with Idiopathic Pulmonary Fibrosis and Other Interstitial Lung Diseases.Biomedicines. 2023 Dec 8;11(12):3257. doi: 10.3390/biomedicines11123257. Biomedicines. 2023. PMID: 38137478 Free PMC article.
-
Clinical Impact of Telomere Length Testing for Interstitial Lung Disease.Chest. 2024 Nov;166(5):1071-1081. doi: 10.1016/j.chest.2024.06.006. Epub 2024 Jun 29. Chest. 2024. PMID: 38950694
-
Interstitial lung disease: a review of classification, etiology, epidemiology, clinical diagnosis, pharmacological and non-pharmacological treatment.Front Med (Lausanne). 2024 Apr 18;11:1296890. doi: 10.3389/fmed.2024.1296890. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38698783 Free PMC article. Review.
-
Polygenic Risk and Rare Variants in Endotypes of Idiopathic Pulmonary Fibrosis.medRxiv [Preprint]. 2025 Jun 4:2025.05.22.25328177. doi: 10.1101/2025.05.22.25328177. medRxiv. 2025. PMID: 40502585 Free PMC article. Preprint.
References
-
- Duckworth, A. et al. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: a mendelian randomisation study. Lancet Respir. Med. 9, 285–294 (2020). - PubMed

