Cellular and molecular mechanisms of Hedgehog signalling
- PMID: 36932157
- PMCID: PMC12140928
- DOI: 10.1038/s41580-023-00591-1
Cellular and molecular mechanisms of Hedgehog signalling
Abstract
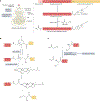
The Hedgehog signalling pathway has crucial roles in embryonic tissue patterning, postembryonic tissue regeneration, and cancer, yet aspects of Hedgehog signal transmission and reception have until recently remained unclear. Biochemical and structural studies surprisingly reveal a central role for lipids in Hedgehog signalling. The signal - Hedgehog protein - is modified by cholesterol and palmitate during its biogenesis, thereby necessitating specialized proteins such as the transporter Dispatched and several lipid-binding carriers for cellular export and receptor engagement. Additional lipid transactions mediate response to the Hedgehog signal, including sterol activation of the transducer Smoothened. Access of sterols to Smoothened is regulated by the apparent sterol transporter and Hedgehog receptor Patched, whose activity is blocked by Hedgehog binding. Alongside these lipid-centric mechanisms and their relevance to pharmacological pathway modulation, we discuss emerging roles of Hedgehog pathway activity in stem cells or their cellular niches, with translational implications for regeneration and restoration of injured or diseased tissues.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures






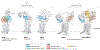
References
-
- Ives PT New mutants report. Drosoph. Inf. Serv 24, 58 (1950).
-
- Lee JJ, von Kessler DP, Parks S & Beachy PA Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71, 33–50 (1992). - PubMed
-
- Nusslein-Volhard C & Wieschaus E Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801 (1980). - PubMed
-
- Dessaud E, McMahon AP & Briscoe J Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489–2503 (2008). - PubMed
-
- Ingham PW & McMahon AP Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15, 3059–3087 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

