Effect of Various Dosing Schedules on the Pharmacokinetics of Oral Semaglutide: A Randomised Trial in Healthy Subjects
- PMID: 36932262
- PMCID: PMC10023024
- DOI: 10.1007/s40262-023-01223-9
Effect of Various Dosing Schedules on the Pharmacokinetics of Oral Semaglutide: A Randomised Trial in Healthy Subjects
Erratum in
-
Correction to: Effect of Various Dosing Schedules on the Pharmacokinetics of Oral Semaglutide: A Randomised Trial in Healthy Subjects.Clin Pharmacokinet. 2023 Jul;62(7):1045-1047. doi: 10.1007/s40262-023-01276-w. Clin Pharmacokinet. 2023. PMID: 37378793 Free PMC article. No abstract available.
Abstract
Background: Prescribing information instructs taking oral semaglutide (a glucagon-like peptide-1 analogue) in the fasting state, followed by a post-dose fasting period of ≥ 30 min. This trial compared the recommended dosing schedule with alternative schedules.
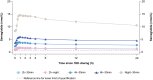
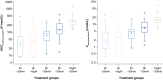
Methods: This was a randomised, single-centre, multiple-dose, open-label, five-armed, parallel-group trial in healthy subjects who received once-daily oral semaglutide (3 mg for 5 days followed by 7 mg for 5 days). Subjects (n = 156) were randomised to five dosing schedules: 2-, 4-, or 6-h pre-dose fast followed by a 30-min post-dose fast (treatment arms: 2 h-30 min, 4-30 min, 6 h-30 min); 2-h pre-dose fast followed by an overnight post-dose fast (treatment arm: 2 h-night); or overnight pre-dose fast followed by a 30-min post-dose fast (reference arm: night-30 min). Semaglutide plasma concentration was measured regularly until 24 h after the 10th dose. Endpoints included area under the semaglutide plasma concentration-time curve during a 24-h interval after the 10th dose (AUC0-24h) (primary endpoint) and maximum observed semaglutide plasma concentration after the 10th dose (Cmax) (secondary endpoint).
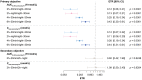
Results: Compared with an overnight pre-dose fast (reference arm: night-30 min), shorter pre-dose fasting times in the 2 h-night, 2 h-30 min, 4 h-30 min, and 6 h-30 min treatment arms resulted in significantly lower semaglutide AUC0-24h and Cmax after the 10th dose (estimated treatment ratio ranges: 0.12-0.43 and 0.11-0.44, respectively; p < 0.0001 for all comparisons). Semaglutide AUC0-24h and Cmax after the 10th dose were similar for the 2 h-30 min and 2 h-night treatment arms.
Conclusion: This trial supports dosing oral semaglutide in accordance with prescribing information, which requires dosing in the fasting state.
Trial registration: ClinicalTrials.gov (NCT04513704); registered August 14, 2020.
Plain language summary
Oral semaglutide is a human glucagon-like peptide-1 analogue that has been approved for the treatment of type 2 diabetes. It has been established that taking oral semaglutide with food or large volumes of water decreases absorption of the drug in the body. Current prescribing information instructs taking oral semaglutide on an empty stomach (known as the fasting state), with 120 mL/4 oz of water, then waiting for at least 30 min before consuming any food, water, or taking other oral medications. This study investigates whether different dosing schedules for oral semaglutide could potentially offer more flexibility to patients in the timing of their oral semaglutide dosing. The trial, conducted in healthy volunteers, compares the dosing schedule described in the prescribing information with different fasting times before (pre-dose) and after (post-dose) taking oral semaglutide during the day or evening, to see if there were any effects on the concentration of drug in the body. Compared to the recommended overnight fasting period, shorter pre-dose fasting periods of 2–6 h with a 30-min post-dose fast considerably reduced semaglutide exposure in the body. Similarly, semaglutide exposure was also reduced with a 2-h pre-dose fast combined with post-dose overnight fasting. These findings further support the current prescribing information, which states that patients should take their oral semaglutide dose after an overnight fast.
© 2023. The Author(s).
Conflict of interest statement
MvH, TBJ, CB, and TAB are employees, and MvH, TBJ, and TAB are shareholders, of Novo Nordisk A/S, the sponsors of this trial. PF is an employee of PAREXEL International.
Figures

References
-
- US Food and Drug Administration. Rybelsus® (semaglutide) tablets: prescribing information; 2023. https://www.accessdata.fda.gov/spl/data/fa74bbc6-5386-425e-8d4a-6151795b.... Accessed March 31, 2022.
-
- Baekdal TA, Breitschaft A, Donsmark M, Maarbjerg SJ, Sondergaard FL, Borregaard J. Effect of various dosing conditions on the pharmacokinetics of oral semaglutide, a human glucagon-like peptide-1 analogue in a tablet formulation. Diabetes Ther. 2021;12(7):1915–1927. doi: 10.1007/s13300-021-01078-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

