Nuclear Translocation Triggered at the Onset of Hearing in Cochlear Inner Hair Cells of Rats and Mice
- PMID: 36932316
- PMCID: PMC10335982
- DOI: 10.1007/s10162-023-00894-2
Nuclear Translocation Triggered at the Onset of Hearing in Cochlear Inner Hair Cells of Rats and Mice
Abstract
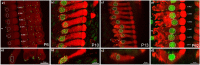
Purpose: Nuclear position is precisely orchestrated during cell division, migration, and maturation of cells and tissues. Here we report a previously unrecognized, programmed movement of the nucleus in rat and mouse cochlear inner hair cells (IHCs) coinciding with the functional maturation of inner hair cells around the onset of hearing.
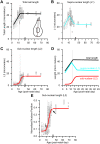
Methods: We measured hair cell length and nuclear position from confocal scans of immunofluorescence-labeled hair cells from whole-mount cochlear preparations throughout post-natal development.
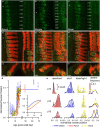
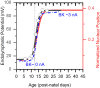
Results: In early post-natal days, the IHC experiences a period of sustained growth, during which the nucleus sits at the very basal pole of the cell, far from the apically located mechano-transducing stereocilia, but close to where synapses with primary afferent and efferent neurons are forming. After IHCs reach their final length, the nucleus moves to occupy a new position half-way along the length of the cell. Nuclear translocation begins in the middle turn, completes throughout the cochlea within 2-3 days, and coincides with the emergence of endolymphatic potential, the acquisition of big-conductance potassium channels (BK), and the onset of acoustic hearing. IHCs cultured in-vitro without endolymphatic potential (EP) do not grow, do not express BK, and do not experience nuclear movement. IHCs cultured in high K+ solutions (to simulate EP) grow but do not experience nuclear movement or acquire BK channels.
Conclusion: Nuclear migration at the onset of hearing is a key step in the morphological maturation of IHCs. Whether this plays a role in functional maturation remains to be explored.
Keywords: Cochlea; Hair cells; Nuclear translocation; Onset of hearing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Biophysical and morphological changes in inner hair cells and their efferent innervation in the ageing mouse cochlea.J Physiol. 2021 Jan;599(1):269-287. doi: 10.1113/JP280256. Epub 2020 Nov 17. J Physiol. 2021. PMID: 33179774 Free PMC article.
-
Gata3 is required for the functional maturation of inner hair cells and their innervation in the mouse cochlea.J Physiol. 2019 Jul;597(13):3389-3406. doi: 10.1113/JP277997. Epub 2019 May 28. J Physiol. 2019. PMID: 31069810 Free PMC article.
-
Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels.Neuroscience. 2006 Sep 15;141(4):1849-60. doi: 10.1016/j.neuroscience.2006.05.057. Epub 2006 Jul 10. Neuroscience. 2006. PMID: 16828974
-
How to build an inner hair cell: challenges for regeneration.Hear Res. 2007 May;227(1-2):3-10. doi: 10.1016/j.heares.2006.12.005. Epub 2006 Dec 16. Hear Res. 2007. PMID: 17258412 Review.
-
The auditory hair cell ribbon synapse: from assembly to function.Annu Rev Neurosci. 2012;35:509-28. doi: 10.1146/annurev-neuro-061010-113705. Annu Rev Neurosci. 2012. PMID: 22715884 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

