Seven-year outcomes following intensive anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration
- PMID: 36932356
- PMCID: PMC10022151
- DOI: 10.1186/s12886-023-02843-2
Seven-year outcomes following intensive anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration
Abstract
Background: Anti-vascular endothelial growth factor (VEGF) therapy is currently the most effective therapy of exudative age-related macular degeneration (AMD). The aim of this study was to assess long-term benefits of intensive aflibercept and ranibizumab anti-VEGF therapy in patients with exudative AMD.
Methods: Two clinical trial sites recruited their original subjects for a re-evaluation 7 years after the baseline visit of the phase-3 Vascular Endothelial Growth Factor (VEGF) Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration (VIEW 2) trial. Forty-seven eyes of 47 patients with AMD originally treated with ranibizumab (14 eyes) or aflibercept (33 eyes) were included.
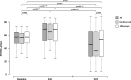
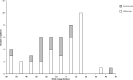
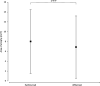
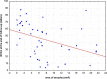
Results: Mean number of injections was 17.8 ± 3.0 during participation in the VIEW 2 trial. Fourteen of 47 (30%) eyes were given additional injections with a mean number of 5.7 ± 4.5 after the trial. At a mean follow-up time of 82 ± 5 months best corrected visual acuity (BCVA) remained stable or improved (≤ 10 letters lost) in 55% of patients in the entire study population, in 43% in the ranibizumab group and in 60% in the aflibercept group. In both groups combined mean BCVA was 54 ± 13 letters at baseline, 65 ± 17 letters at the end of the intensive phase and 45 ± 25 letters at the end of follow-up. There was no statistically significant difference in BCVA between the two groups at baseline (p = 0.88) and at the end of follow-up (p = 0.40). Macular atrophy was observed in 96% of eyes, average area was 7.22 ± 6.31 mm2 with no statistically significant difference between groups (p = 0.47). Correlation between BCVA at end-of-follow-up and the area of atrophy was significant (p < 0.001). At the end of follow-up, fluid was detected in 7 of 47 eyes (15%) indicating disease activity.
Conclusion: Long-term efficacy of aflibercept and ranibizumab was largely consistent. Following a two-year intensive therapy with as-needed regimen, BCVA was maintained or improved in almost half of the patients and in the ranibizumab group and more than half of the patients in the aflibercept group with very few injections. In a remarkable proportion of eyes, BCVA declined severely which underlines the need for long-term follow-ups and may indicate a more prolonged intensive therapy.
Trial registrations: VIEW 2 study: ClinicalTrials.gov ID: NCT00637377, date of registration: March 18, 2008. Long-term follow-up: IRB nr.: SE RKEB 168/2022, ClinicalTrials.gov ID: NCT05678517, date of registration: December 28, 2022, retrospectively registered.
Keywords: AMD; Aflibercept; Age-related macular degeneration; Anti-VEGF; Exudative; Long-term treatment; Macula; Ranibizumab; Retina; Wet.
© 2023. The Author(s).
Conflict of interest statement
RL: Has received investigator fees from Bayer and Novartis, not related to this work.
MS: Has received investigator fees from Allergan, Bayer, Novartis, and Roche, has served as an advisory board member for Novartis and Roche, and acted as consultant for AbbVie, not related to this work.
GLS: No conflicting interests.
KK: No conflicting interests.
AA: Has received investigator fees from Bayer and Novartis, not related to this work.
LE: Has received investigator fees from Bayer and Novartis, not related to this work.
GP: Has received investigator fees from Bayer and Novartis, not related to this work.
GB: Has received investigator fees from Bayer and Novartis, not related to this work.
AS: Has received investigator fees from Bayer and Novartis, not related to this work.
AB: Has received investigator fees from Bayer and Novartis, not related to this work.
IK: Has received investigator fees from Bayer and Novartis, not related to this work.
MDR: Has received investigator fees from Bayer and Novartis, not related to this work.
ZZN: Has received investigator fees from Bayer and Novartis, not related to this work.
AP: Has received investigator fees from Allergan, Bayer and Novartis, has served as an advisory board member for Bayer and Novartis.
No other conflicting relationship relevant to this manuscript exists for any of the authors.
Figures
Similar articles
-
Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial).Br J Ophthalmol. 2014 Jul;98(7):951-5. doi: 10.1136/bjophthalmol-2013-304736. Epub 2014 Feb 11. Br J Ophthalmol. 2014. PMID: 24518078 Free PMC article. Clinical Trial.
-
Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP).Ophthalmology. 2013 Nov;120(11):2292-9. doi: 10.1016/j.ophtha.2013.03.046. Epub 2013 May 3. Ophthalmology. 2013. PMID: 23642856 Clinical Trial.
-
Real-world 10-year experiences with intravitreal treatment with ranibizumab and aflibercept for neovascular age-related macular degeneration.Acta Ophthalmol. 2020 Mar;98(2):132-138. doi: 10.1111/aos.14183. Epub 2019 Jul 8. Acta Ophthalmol. 2020. PMID: 31282617
-
Efficacy and safety of brolucizumab in age-related macular degeneration: A systematic review of real-world studies.Acta Ophthalmol. 2023 Mar;101(2):123-139. doi: 10.1111/aos.15242. Epub 2022 Sep 18. Acta Ophthalmol. 2023. PMID: 36117281
-
Aflibercept in wet AMD: specific role and optimal use.Drug Des Devel Ther. 2013 Aug 5;7:711-22. doi: 10.2147/DDDT.S40215. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 23990705 Free PMC article. Review.
Cited by
-
Recent Advances in Imaging Macular Atrophy for Late-Stage Age-Related Macular Degeneration.Diagnostics (Basel). 2023 Dec 10;13(24):3635. doi: 10.3390/diagnostics13243635. Diagnostics (Basel). 2023. PMID: 38132220 Free PMC article. Review.
-
VEGF in Tears as a Biomarker for Exudative Age-Related Macular Degeneration: Molecular Dynamics in a Mouse Model and Human Samples.Int J Mol Sci. 2025 Apr 18;26(8):3855. doi: 10.3390/ijms26083855. Int J Mol Sci. 2025. PMID: 40332529 Free PMC article.
-
Outcomes of Anti-VEGF Therapy in Eyes with Diabetic Macular Edema, Vein Occlusion-Related Macular Edema, and Neovascular Age-Related Macular Degeneration: A Systematic Review.Clin Ophthalmol. 2024 Dec 17;18:3837-3851. doi: 10.2147/OPTH.S489114. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39717563 Free PMC article. Review.
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

