Cryptococcus neoformans, a global threat to human health
- PMID: 36932414
- PMCID: PMC10020775
- DOI: 10.1186/s40249-023-01073-4
Cryptococcus neoformans, a global threat to human health
Abstract
Background: Emerging fungal pathogens pose important threats to global public health. The World Health Organization has responded to the rising threat of traditionally neglected fungal infections by developing a Fungal Priority Pathogens List (FPPL). Taking the highest-ranked fungal pathogen in the FPPL, Cryptococcus neoformans, as a paradigm, we review progress made over the past two decades on its global burden, its clinical manifestation and management of cryptococcal infection, and its antifungal resistance. The purpose of this review is to drive research efforts to improve future diagnoses, therapies, and interventions associated with fungal infections.
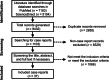
Methods: We first reviewed trends in the global burden of HIV-associated cryptococcal infection, mainly based on a series of systematic studies. We next conducted scoping reviews in accordance with the guidelines described in the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for Scoping Reviews using PubMed and ScienceDirect with the keyword Cryptococcus neoformans to identify case reports of cryptococcal infections published since 2000. We then reviewed recent updates on the diagnosis and antifungal treatment of cryptococcal infections. Finally, we summarized knowledge regarding the resistance and tolerance of C. neoformans to approved antifungal drugs.
Results: There has been a general reduction in the estimated global burden of HIV-associated cryptococcal meningitis since 2009, probably due to improvements in highly active antiretroviral therapies. However, cryptococcal meningitis still accounts for 19% of AIDS-related deaths annually. The incidences of CM in Europe and North America and the Latin America region have increased by approximately two-fold since 2009, while other regions showed either reduced or stable numbers of cases. Unfortunately, diagnostic and treatment options for cryptococcal infections are limited, and emerging antifungal resistance exacerbates the public health burden.
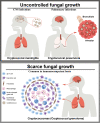
Conclusion: The rising threat of C. neoformans is compounded by accumulating evidence for its ability to infect immunocompetent individuals and the emergence of antifungal-resistant variants. Emphasis should be placed on further understanding the mechanisms of pathogenicity and of antifungal resistance and tolerance. The development of novel management strategies through the identification of new drug targets and the discovery and optimization of new and existing diagnostics and therapeutics are key to reducing the health burden.
Keywords: Antifungal resistance; Antifungal tolerance; Cryptococcal meningitis; Cryptococcus neoformans; Fungal infections.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

