Tumor cell integrin β4 and tumor stroma E-/P-selectin cooperatively regulate tumor growth in vivo
- PMID: 36932441
- PMCID: PMC10022201
- DOI: 10.1186/s13045-023-01413-9
Tumor cell integrin β4 and tumor stroma E-/P-selectin cooperatively regulate tumor growth in vivo
Abstract
Background: The immunological composition of the tumor microenvironment has a decisive influence on the biological course of cancer and is therefore of profound clinical relevance. In this study, we analyzed the cooperative effects of integrin β4 (ITGB4) on tumor cells and E-/P-selectin on endothelial cells within the tumor stroma for regulating tumor growth by shaping the local and systemic immune environment.
Methods: We used several preclinical mouse models for different solid human cancer types (xenograft and syngeneic) to explore the role of ITGB4 (shRNA-mediated knockdown in tumor cells) and E-/P-selectins (knockout in mice) for tumor growth; effects on apoptosis, proliferation and intratumoral signaling pathways were determined by histological and biochemical methods and 3D in vitro experiments; changes in the intratumoral and systemic immune cell composition were determined by flow cytometry and immunohistochemistry; chemokine levels and their attracting potential were measured by ELISA and 3D invasion assays.
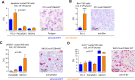
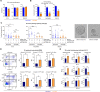
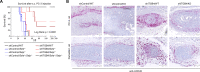
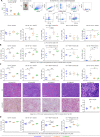
Results: We observed a very robust synergism between ITGB4 and E-/P-selectin for the regulation of tumor growth, accompanied by an increased recruitment of CD11b+ Gr-1Hi cells with low granularity (i.e., myeloid-derived suppressor cells, MDSCs) specifically into ITGB4-depleted tumors. ITGB4-depleted tumors undergo apoptosis and actively attract MDSCs, well-known to promote tumor growth in several cancers, via increased secretion of different chemokines. MDSC trafficking into tumors crucially depends on E-/P-selectin expression. Analyses of clinical samples confirmed an inverse relationship between ITGB4 expression in tumors and number of tumor-infiltrating leukocytes.
Conclusions: These findings suggest a distinct vulnerability of ITGB4Lo tumors for MDSC-directed immunotherapies.
Keywords: Anoikis; Chemoattraction; E-selectin; Integrin β4; Myeloid-derived suppressor cell; P-selectin; Tumor-infiltrating leukocyte.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Gebauer F, Wicklein D, Stubke K, Nehmann N, Schmidt A, Salamon J, Peldschus K, Nentwich MF, Adam G, Tolstonog G, Bockhorn M, Izbicki JR, Wagener C, Schumacher U. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp–/rag2– mice. Gut. 2013;62:741–750. doi: 10.1136/gutjnl-2011-300629. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

