Impact of peri-procedural management of direct oral anticoagulants on pocket haematoma after cardiac electronic device implantation: the StimAOD multicentre prospective study
- PMID: 36932714
- PMCID: PMC10227661
- DOI: 10.1093/europace/euad057
Impact of peri-procedural management of direct oral anticoagulants on pocket haematoma after cardiac electronic device implantation: the StimAOD multicentre prospective study
Abstract
Aims: The study aims to investigate the impact of direct oral anticoagulant (DOAC) management on the incidence of pocket haematoma in patients undergoing pacemaker or implantable cardioverter-defibrillator implantation.
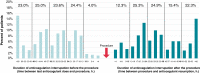
Methods and results: All consecutive patients receiving DOAC and undergoing cardiac electronic device implantation were included in a large multicentre prospective observational study (NCT03879473). The primary endpoint was clinically relevant haematoma within 30 days after implantation. Overall, 789 patients were enrolled [median age 80 (IQR 72-85) years old, 36.4% women, median CHA2DS2-VASc score 4 (IQR 0-8)], of which 632 (80.1%) received a pacemaker implantation. Antiplatelet therapy was combined with DOAC in 146 patients (18.5%). Direct oral anticoagulants (DOACs) were interrupted 52 (IQR 37-62) h before the procedure and resumed 31 (IQR 21-47) h later. Ninety-six percent of the patients had at least 12 h DOAC interruption before the procedure, and 78% had at least 12 h DOAC interruption after the procedure. Overall, anticoagulation was interrupted for 72 (IQR 48-96) h. Pre- or post-procedural heparin bridging was used in 8.2% and 3.9%, respectively. Timing of DOAC interruption of resumption was not associated with clinically relevant haematoma. Clinically relevant haematoma occurred in 26 patients (3.3%), and thromboembolic events occurred in 5 patients (0.6%).
Conclusion: In this large real-life registry where most patients had DOAC interruption, clinically relevant haematoma was rare. Despite DOAC interruption and high CHA2DS2-VASc score, thromboembolic events occurred seldomly, highlighting that bleeding exceeds thromboembolic risk in this peri-procedural period. Future research is needed to identify risk factors for clinically relevant haematoma and meaningfully guide clinicians in optimizing DOAC management.
Keywords: Direct oral anticoagulant; Implantable cardioverter–defibrillators; Pacemaker; Pocket haematoma.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.C.M. declares fees from Alliance BMS–Pfizer, Bayer, Boehringer Ingelheim, Abbott, and Novartis. J.M.S. declares fees from BMS–Pfizer, Bayer, and Boehringer Ingelheim. V.A. declares fees from Pfizer and Alnylam. W.A. served as a speaker or a member of a speaker’s bureau for Abbott, Bayer HealthCare Pharmaceuticals, Biotronik, Boston Scientific, Bristol-Myers Squibb, MicroPort, and Medtronic, Inc. E.G. declares consulting fees from MicroPort and Medtronic. N.L. declares fees from BMS–Pfizer and Bayer. S.B. is consultant for Medtronic, Boston Scientific, MicroPort, and Zoll. R.G. received research grants from Abbott, Medtronic, Boston Scientific, and MicroPort and consulting fees from Abbott and Boston Scientific. A.G. reports personal fees from Aguettant, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, LFB, and Sanofi, outside the submitted work. E.M. received research grants from Abbott, Medtronic, Boston Scientific, Biotronik, and MicroPort and declares consulting fees from Abbott, Boston Scientific, Medtronic, Zoll, and Bayer. O.W., A.B., J.L., A.M., P.R., and F.T. declare no conflict of interest.
Figures
Similar articles
-
Interrupted versus uninterrupted anticoagulation for cardiac rhythm management device insertion.Cochrane Database Syst Rev. 2025 Jan 28;1(1):CD013816. doi: 10.1002/14651858.CD013816.pub2. Cochrane Database Syst Rev. 2025. PMID: 39873294
-
Perioperative direct oral anticoagulant management during cardiac implantable electronic device surgery: an updated systematic review and meta-analysis.J Interv Card Electrophysiol. 2025 Jun;68(4):845-856. doi: 10.1007/s10840-024-01947-z. Epub 2024 Nov 15. J Interv Card Electrophysiol. 2025. PMID: 39546144
-
Use of Direct Oral Anticoagulants Following Cardiac Implantable Electronic Device Placement.Pacing Clin Electrophysiol. 2025 Aug;48(8):859-869. doi: 10.1111/pace.70016. Epub 2025 Jul 31. Pacing Clin Electrophysiol. 2025. PMID: 40743214 Review.
-
Interrupted versus uninterrupted anticoagulation therapy for catheter ablation in adults with arrhythmias.Cochrane Database Syst Rev. 2021 Oct 21;10(10):CD013504. doi: 10.1002/14651858.CD013504.pub2. Cochrane Database Syst Rev. 2021. PMID: 34674223 Free PMC article.
-
Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide.J Thromb Haemost. 2008 Oct;6(10):1615-21. doi: 10.1111/j.1538-7836.2008.03080.x. Epub 2008 Jul 12. J Thromb Haemost. 2008. PMID: 18638011
Cited by
-
Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios.J Clin Med. 2023 Sep 13;12(18):5955. doi: 10.3390/jcm12185955. J Clin Med. 2023. PMID: 37762897 Free PMC article. Review.
-
Microporous polysaccharide hemospheres for reducing pocket hematomas after cardiac device implantation in patients on antithrombotic therapy.J Arrhythm. 2024 Aug 13;40(5):1150-1157. doi: 10.1002/joa3.13130. eCollection 2024 Oct. J Arrhythm. 2024. PMID: 39416235 Free PMC article.
-
Intra-pocket ultrasound-guided axillary vein puncture vs. cephalic vein cutdown for cardiac electronic device implantation: the ACCESS trial.Eur Heart J. 2023 Dec 7;44(46):4847-4858. doi: 10.1093/eurheartj/ehad629. Eur Heart J. 2023. PMID: 37832512 Free PMC article. Clinical Trial.
References
-
- Birnie DH, Healey JS, Essebag V. Management of anticoagulation around pacemaker and defibrillator surgery. Circulation 2014;129:2062–5. - PubMed
-
- Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu Bet al. . Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol 2016;67:1300–8. - PubMed
-
- Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–77. - PubMed
-
- Sridhar ARM, Yarlagadda V, Yeruva MR, Kanmanthareddy A, Vallakati A, Dawn Bet al. . Impact of haematoma after pacemaker and CRT device implantation on hospitalization costs, length of stay, and mortality: a population-based study. Europace 2015;17:1548–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical