Heterologous Assembly of Pleomorphic Bacterial Microcompartment Shell Architectures Spanning the Nano- to Microscale
- PMID: 36932732
- PMCID: PMC10330516
- DOI: 10.1002/adma.202212065
Heterologous Assembly of Pleomorphic Bacterial Microcompartment Shell Architectures Spanning the Nano- to Microscale
Abstract
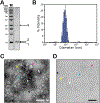
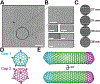
Many bacteria use protein-based organelles known as bacterial microcompartments (BMCs) to organize and sequester sequential enzymatic reactions. Regardless of their specialized metabolic function, all BMCs are delimited by a shell made of multiple structurally redundant, yet functionally diverse, hexameric (BMC-H), pseudohexameric/trimeric (BMC-T), or pentameric (BMC-P) shell protein paralogs. When expressed without their native cargo, shell proteins have been shown to self-assemble into 2D sheets, open-ended nanotubes, and closed shells of ≈40 nm diameter that are being developed as scaffolds and nanocontainers for applications in biotechnology. Here, by leveraging a strategy for affinity-based purification, it is demonstrated that a wide range of empty synthetic shells, many differing in end-cap structures, can be derived from a glycyl radical enzyme-associated microcompartment. The range of pleomorphic shells observed, which span ≈2 orders of magnitude in size from ≈25 nm to ≈1.8 µm, reveal the remarkable plasticity of BMC-based biomaterials. In addition, new capped nanotube and nanocone morphologies are observed that are consistent with a multicomponent geometric model in which architectural principles are shared among asymmetric carbon, viral protein, and BMC-based structures.
Keywords: bacterial microcompartments; fullerenes; nanocones; nanotubes; self-assembly; synthetic biology.
© 2023 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Figures



Similar articles
-
Modulation of Hybrid GRM2-type Bacterial Microcompartment Shells through BMC-H Shell Protein Fusion and Incorporation of Non-native BMC-T Shell Proteins.ACS Synth Biol. 2023 Nov 17;12(11):3275-3286. doi: 10.1021/acssynbio.3c00281. Epub 2023 Nov 8. ACS Synth Biol. 2023. PMID: 37937366
-
A designed bacterial microcompartment shell with tunable composition and precision cargo loading.Metab Eng. 2019 Jul;54:286-291. doi: 10.1016/j.ymben.2019.04.011. Epub 2019 May 7. Metab Eng. 2019. PMID: 31075444 Free PMC article.
-
Programmed loading and rapid purification of engineered bacterial microcompartment shells.Nat Commun. 2018 Jul 23;9(1):2881. doi: 10.1038/s41467-018-05162-z. Nat Commun. 2018. PMID: 30038362 Free PMC article.
-
Dynamic structural determinants in bacterial microcompartment shells.Curr Opin Microbiol. 2024 Aug;80:102497. doi: 10.1016/j.mib.2024.102497. Epub 2024 Jun 21. Curr Opin Microbiol. 2024. PMID: 38909546 Review.
-
Bio-engineering of bacterial microcompartments: a mini review.Biochem Soc Trans. 2019 Jun 28;47(3):765-777. doi: 10.1042/BST20170564. Epub 2019 Jun 24. Biochem Soc Trans. 2019. PMID: 31235547 Review.
Cited by
-
Modeling bacterial microcompartment architectures for enhanced cyanobacterial carbon fixation.Front Plant Sci. 2024 Feb 15;15:1346759. doi: 10.3389/fpls.2024.1346759. eCollection 2024. Front Plant Sci. 2024. PMID: 38425792 Free PMC article. Review.
-
Robust Synthetic Biology Toolkit to Advance Carboxysome Study and Redesign.ACS Synth Biol. 2025 Jun 20;14(6):2219-2229. doi: 10.1021/acssynbio.5c00144. Epub 2025 Jun 9. ACS Synth Biol. 2025. PMID: 40488673 Free PMC article.
-
A robust synthetic biology toolkit to advance carboxysome study and redesign.bioRxiv [Preprint]. 2024 Oct 8:2024.10.08.617227. doi: 10.1101/2024.10.08.617227. bioRxiv. 2024. Update in: ACS Synth Biol. 2025 Jun 20;14(6):2219-2229. doi: 10.1021/acssynbio.5c00144. PMID: 39416180 Free PMC article. Updated. Preprint.
-
Bacterial microcompartments as a next-generation metabolic engineering tool: utilizing nature's solution for confining challenging catabolic pathways.Biochem Soc Trans. 2024 Jun 26;52(3):997-1010. doi: 10.1042/BST20230229. Biochem Soc Trans. 2024. PMID: 38813858 Free PMC article. Review.
-
Chaotrope-Based Approach for Rapid In Vitro Assembly and Loading of Bacterial Microcompartment Shells.ACS Nano. 2025 Apr 1;19(12):11913-11923. doi: 10.1021/acsnano.4c15538. Epub 2025 Mar 20. ACS Nano. 2025. PMID: 40113598 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

