Heterologous Assembly of Pleomorphic Bacterial Microcompartment Shell Architectures Spanning the Nano- to Microscale
- PMID: 36932732
- PMCID: PMC10330516
- DOI: 10.1002/adma.202212065
Heterologous Assembly of Pleomorphic Bacterial Microcompartment Shell Architectures Spanning the Nano- to Microscale
Abstract
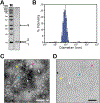
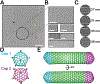
Many bacteria use protein-based organelles known as bacterial microcompartments (BMCs) to organize and sequester sequential enzymatic reactions. Regardless of their specialized metabolic function, all BMCs are delimited by a shell made of multiple structurally redundant, yet functionally diverse, hexameric (BMC-H), pseudohexameric/trimeric (BMC-T), or pentameric (BMC-P) shell protein paralogs. When expressed without their native cargo, shell proteins have been shown to self-assemble into 2D sheets, open-ended nanotubes, and closed shells of ≈40 nm diameter that are being developed as scaffolds and nanocontainers for applications in biotechnology. Here, by leveraging a strategy for affinity-based purification, it is demonstrated that a wide range of empty synthetic shells, many differing in end-cap structures, can be derived from a glycyl radical enzyme-associated microcompartment. The range of pleomorphic shells observed, which span ≈2 orders of magnitude in size from ≈25 nm to ≈1.8 µm, reveal the remarkable plasticity of BMC-based biomaterials. In addition, new capped nanotube and nanocone morphologies are observed that are consistent with a multicomponent geometric model in which architectural principles are shared among asymmetric carbon, viral protein, and BMC-based structures.
Keywords: bacterial microcompartments; fullerenes; nanocones; nanotubes; self-assembly; synthetic biology.
© 2023 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

