The chaperone system in cancer therapies: Hsp90
- PMID: 36933095
- PMCID: PMC10079721
- DOI: 10.1007/s10735-023-10119-8
The chaperone system in cancer therapies: Hsp90
Abstract
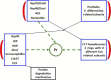
The chaperone system (CS) of an organism is composed of molecular chaperones, chaperone co-factors, co-chaperones, and chaperone receptors and interactors. It is present throughout the body but with distinctive features for each cell and tissue type. Previous studies pertaining to the CS of the salivary glands have determined the quantitative and distribution patterns for several members, the chaperones, in normal and diseased glands, focusing on tumors. Chaperones are cytoprotective, but can also be etiopathogenic agents causing diseases, the chaperonopathies. Some chaperones such as Hsp90 potentiate tumor growth, proliferation, and metastasization. Quantitative data available on this chaperone in salivary gland tissue with inflammation, and benign and malignant tumors suggest that assessing tissue Hsp90 levels and distribution patterns is useful for differential diagnosis-prognostication, and patient follow up. This, in turn, will reveal clues for developing specific treatment centered on the chaperone, for instance by inhibiting its pro-carcinogenic functions (negative chaperonotherapy). Here, we review data on the carcinogenic mechanisms of Hsp90 and their inhibitors. Hsp90 is the master regulator of the PI3K-Akt-NF-kB axis that promotes tumor cell proliferation and metastasization. We discuss pathways and interactions involving these molecular complexes in tumorigenesis and review Hsp90 inhibitors that have been tested in search of an efficacious anti-cancer agent. This targeted therapy deserves extensive investigation in view of its theoretical potential and some positive practical results and considering the need of novel treatments for tumors of the salivary glands as well as other tissues.
Keywords: Akt; Chaperone system; Chaperonopathies; Hsp90; Molecular chaperone; NF-kB; Negative chaperonotherapy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

