Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer's disease
- PMID: 36933144
- PMCID: PMC10400494
- DOI: 10.1007/s11357-023-00773-x
Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer's disease
Abstract
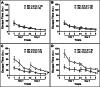
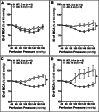
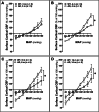
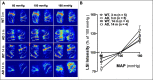
Alzheimer's disease (AD) is a global healthcare crisis. The TgF344-AD rat is an AD model exhibiting age-dependent AD pathological hallmarks. We confirmed that AD rats developed cognitive deficits at 6 months without alteration of any other major biophysical parameters. We longitudinally characterized cerebral hemodynamics in AD rats at 3, 4, 6, and 14 months. The myogenic responses of the cerebral arteries and arterioles were impaired at 4 months of age in the AD rats. Consistent with the ex vivo results, the AD rat exhibited poor autoregulation of surface and deep cortical cerebral blood flow 2 months preceding cognitive decline. The dysfunction of cerebral hemodynamics in AD is exacerbated with age associated with reduced cerebral perfusion. Further, abolished cell contractility contributes to cerebral hemodynamics imbalance in AD. This may be attributed to enhanced ROS production, reduced mitochondrial respiration and ATP production, and disrupted actin cytoskeleton in cerebral vascular contractile cells.
Keywords: Alzheimer’s disease; Autoregulation; Cognitive impairments; Hemodynamics; Myogenic response; Vascular smooth muscle cells.
© 2023. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Early functional connectivity deficits and progressive microstructural alterations in the TgF344-AD rat model of Alzheimer's Disease: A longitudinal MRI study.Neurobiol Dis. 2019 Apr;124:93-107. doi: 10.1016/j.nbd.2018.11.010. Epub 2018 Nov 13. Neurobiol Dis. 2019. PMID: 30445024
-
Sexual dimorphism in the peripheral metabolic homeostasis and behavior in the TgF344-AD rat model of Alzheimer's disease.Aging Cell. 2023 Jul;22(7):e13854. doi: 10.1111/acel.13854. Epub 2023 Apr 24. Aging Cell. 2023. PMID: 37095621 Free PMC article.
-
Prodromal neuroinflammatory, cholinergic and metabolite dysfunction detected by PET and MRS in the TgF344-AD transgenic rat model of AD: a collaborative multi-modal study.Theranostics. 2021 May 3;11(14):6644-6667. doi: 10.7150/thno.56059. eCollection 2021. Theranostics. 2021. PMID: 34093845 Free PMC article.
-
Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer's disease.J Alzheimers Dis. 2012;32(3):553-67. doi: 10.3233/JAD-2012-120793. J Alzheimers Dis. 2012. PMID: 22842871 Review.
-
Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies.Prog Neurobiol. 2013 Sep;108:21-43. doi: 10.1016/j.pneurobio.2013.06.004. Epub 2013 Jul 11. Prog Neurobiol. 2013. PMID: 23850509 Review.
Cited by
-
Nodal degree centrality in the default mode-like network of the TgF344-AD Alzheimer's disease rat model as a measure of early network alterations.NPJ Aging. 2024 Jun 20;10(1):29. doi: 10.1038/s41514-024-00151-7. NPJ Aging. 2024. PMID: 38902224 Free PMC article.
-
A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer's Disease.Int J Mol Sci. 2025 Mar 8;26(6):2433. doi: 10.3390/ijms26062433. Int J Mol Sci. 2025. PMID: 40141075 Free PMC article.
-
Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling.Geroscience. 2023 Oct;45(5):2909-2926. doi: 10.1007/s11357-023-00841-2. Epub 2023 Jun 16. Geroscience. 2023. PMID: 37326915 Free PMC article.
-
Depth-Dependent Contributions of Various Vascular Zones to Cerebral Autoregulation and Functional Hyperemia: An In-Silico Analysis.bioRxiv [Preprint]. 2024 Oct 11:2024.10.07.616950. doi: 10.1101/2024.10.07.616950. bioRxiv. 2024. Update in: PLoS One. 2025 May 19;20(5):e0321053. doi: 10.1371/journal.pone.0321053. PMID: 39416222 Free PMC article. Updated. Preprint.
-
Quantification and correlation of amyloid-β plaque load, glial activation, GABAergic interneuron numbers, and cognitive decline in the young TgF344-AD rat model of Alzheimer's disease.Front Aging Neurosci. 2025 Feb 12;17:1542229. doi: 10.3389/fnagi.2025.1542229. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40013092 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

