The vascular gene Apold1 is dispensable for normal development but controls angiogenesis under pathological conditions
- PMID: 36933174
- PMCID: PMC10328887
- DOI: 10.1007/s10456-023-09870-z
The vascular gene Apold1 is dispensable for normal development but controls angiogenesis under pathological conditions
Abstract
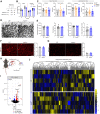
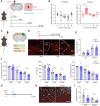
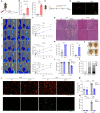
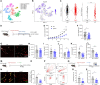
The molecular mechanisms of angiogenesis have been intensely studied, but many genes that control endothelial behavior and fate still need to be described. Here, we characterize the role of Apold1 (Apolipoprotein L domain containing 1) in angiogenesis in vivo and in vitro. Single-cell analyses reveal that - across tissues - the expression of Apold1 is restricted to the vasculature and that Apold1 expression in endothelial cells (ECs) is highly sensitive to environmental factors. Using Apold1-/- mice, we find that Apold1 is dispensable for development and does not affect postnatal retinal angiogenesis nor alters the vascular network in adult brain and muscle. However, when exposed to ischemic conditions following photothrombotic stroke as well as femoral artery ligation, Apold1-/- mice display dramatic impairments in recovery and revascularization. We also find that human tumor endothelial cells express strikingly higher levels of Apold1 and that Apold1 deletion in mice stunts the growth of subcutaneous B16 melanoma tumors, which have smaller and poorly perfused vessels. Mechanistically, Apold1 is activated in ECs upon growth factor stimulation as well as in hypoxia, and Apold1 intrinsically controls EC proliferation but not migration. Our data demonstrate that Apold1 is a key regulator of angiogenesis in pathological settings, whereas it does not affect developmental angiogenesis, thus making it a promising candidate for clinical investigation.
Keywords: Angiogenesis; EC proliferation; Endothelial cell; Hindlimb ischemia; Stroke; Tumor.
© 2023. The Author(s).
Conflict of interest statement
The authors disclose no financial or non-financial interests related to this work.
Figures

References
-
- Nishijima T, Torres-Aleman I, Soya H. Chapter 11 - Exercise and cerebrovascular plasticity. In: Masamoto K, Hirase H, Yamada K, editors. Progress in Brain Research. Elsevier; 2016. pp. 243–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

