Anti-TIGIT therapies for solid tumors: a systematic review
- PMID: 36933320
- PMCID: PMC10030909
- DOI: 10.1016/j.esmoop.2023.101184
Anti-TIGIT therapies for solid tumors: a systematic review
Abstract
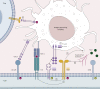
Programmed death-ligand 1[PD-(L)1], cytotoxic T-lymphocyte associated protein 4 (CTLA-4), and lymphocyte-activation gene 3 (LAG-3) inhibitors are recent breakthroughs in cancer treatment, however not all patients benefit from it. Thus new therapies are under investigation, such as anti-TIGIT [anti-T-cell immunoreceptor with immunoglobulin (Ig) and immunoreceptor tyrosine-based inhibitory motif domains] antibodies. TIGIT is an immune checkpoint inhibiting lymphocyte T cells by several mechanisms. In vitro models showed its inhibition could restore antitumor response. Furthermore, its association with anti-PD-(L)1 therapies could synergistically improve survival. We carried out a review of the clinical trial about TIGIT referenced in the PubMed database, finding three published clinical trials on anti-TIGIT therapies. Vibostolimab was evaluated in a phase I alone or in combination with pembrolizumab. The combination had an objective response rate of 26% in patients with a non-small-cell lung cancer (NSCLC) naïve of anti-programmed cell death protein 1 (anti-PD-1). Etigilimab was tested in a phase I alone or in combination with nivolumab, but the study was stopped due to business reasons. In the phase II CITYSCAPE trial, tiragolumab demonstrated higher objective response rate and progression-free survival in combination with atezolizumab than atezolizumab alone in advanced PD-L1-high NSCLC. The ClinicalTrials.gov database references 70 trials of anti-TIGIT in patients with cancer, 47 of them with ongoing recruitment. Only seven were phase III, including five about patients with NSCLC, mostly with combination therapy. Data from phase I-II trials highlighted that targeting TIGIT represents a safe therapeutic approach, with an acceptable toxicity profile maintained when adding anti-PD-(L)1 antibodies. Frequent adverse events were pruritus, rash, and fatigue. Grade 3-4 adverse events were reported in nearly one in three patients. Anti-TIGIT antibodies are under development as a novel immunotherapy approach. A promising research area includes the combination with anti-PD-1 therapies in advanced NSCLCs.
Keywords: TIGIT; immune therapy; lung cancer; tiragolumab.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure FB declares no personal financial interests (since August 2021); institutional financial interests with AbbVie, ACEA, Amgen, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Boehringer–Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis and Takeda; nonfinancial interests as the principal investigator for Astra-Zeneca, BMS, Innate Pharma, Merck, Pierre Fabre and F. Hoffmann-La Roche, Ltd-sponsored trials (or investigator-sponsored trails). The other authors have no conflicts of interest to declare.
Figures
References
-
- Blackhall F., Jao K., Greillier L., et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16(9):1547–1558. - PubMed
-
- Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

