Oxoglutarate dehydrogenase complex controls glutamate-mediated neuronal death
- PMID: 36933393
- PMCID: PMC10031542
- DOI: 10.1016/j.redox.2023.102669
Oxoglutarate dehydrogenase complex controls glutamate-mediated neuronal death
Abstract
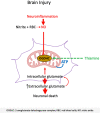
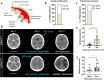
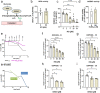
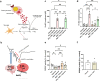
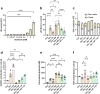
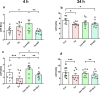
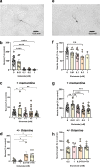
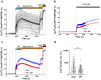
Brain injury is accompanied by neuroinflammation, accumulation of extracellular glutamate and mitochondrial dysfunction, all of which cause neuronal death. The aim of this study was to investigate the impact of these mechanisms on neuronal death. Patients from the neurosurgical intensive care unit suffering aneurysmal subarachnoid hemorrhage (SAH) were recruited retrospectively from a respective database. In vitro experiments were performed in rat cortex homogenate, primary dissociated neuronal cultures, B35 and NG108-15 cell lines. We employed methods including high resolution respirometry, electron spin resonance, fluorescent microscopy, kinetic determination of enzymatic activities and immunocytochemistry. We found that elevated levels of extracellular glutamate and nitric oxide (NO) metabolites correlated with poor clinical outcome in patients with SAH. In experiments using neuronal cultures we showed that the 2-oxoglutarate dehydrogenase complex (OGDHC), a key enzyme of the glutamate-dependent segment of the tricarboxylic acid (TCA) cycle, is more susceptible to the inhibition by NO than mitochondrial respiration. Inhibition of OGDHC by NO or by succinyl phosphonate (SP), a highly specific OGDHC inhibitor, caused accumulation of extracellular glutamate and neuronal death. Extracellular nitrite did not substantially contribute to this NO action. Reactivation of OGDHC by its cofactor thiamine (TH) reduced extracellular glutamate levels, Ca2+ influx into neurons and cell death rate. Salutary effect of TH against glutamate toxicity was confirmed in three different cell lines. Our data suggest that the loss of control over extracellular glutamate, as described here, rather than commonly assumed impaired energy metabolism, is the critical pathological manifestation of insufficient OGDHC activity, leading to neuronal death.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

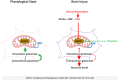
References
-
- Akhtar M.W., Sanz-Blasco S., Dolatabadi N., Parker J., Chon K., Lee M.S., Soussou W., McKercher S.R., Ambasudhan R., Nakamura T., Lipton S.A. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016;7 doi: 10.1038/ncomms10242. - DOI - PMC - PubMed
-
- Sanchez-Padilla J., Guzman J.N., Ilijic E., Kondapalli J., Galtieri D.J., Yang B., Schieber S., Oertel W., Wokosin D., Schumacker P.T., Surmeier D.J. Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat. Neurosci. 2014;17:832–840. doi: 10.1038/nn.3717. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

