Oral polymicrobial communities: Assembly, function, and impact on diseases
- PMID: 36933557
- PMCID: PMC10101935
- DOI: 10.1016/j.chom.2023.02.009
Oral polymicrobial communities: Assembly, function, and impact on diseases
Abstract
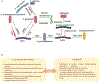
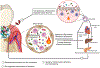
Oral microbial communities assemble into complex spatial structures. The sophisticated physical and chemical signaling systems underlying the community enable their collective functional regulation as well as the ability to adapt by integrating environmental information. The combined output of community action, as shaped by both intra-community interactions and host and environmental variables, dictates homeostatic balance or dysbiotic disease such as periodontitis and dental caries. Oral polymicrobial dysbiosis also exerts systemic effects that adversely affect comorbidities, in part due to ectopic colonization of oral pathobionts in extra-oral tissues. Here, we review new and emerging concepts that explain the collective functional properties of oral polymicrobial communities and how these impact health and disease both locally and systemically.
Keywords: caries; dysbiosis; inflammation; oral microbiome; periodontitis; spatial structure; systemic comorbidities.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.H. is inventor of a patent that describes the use of complement inhibitors in periodontal disease (“Methods of Treating or Preventing Periodontitis and Diseases Associated with Periodontitis”; patent no. 10,668,135). H.K. is inventor of a patent titled “Iron Oxide Nanoparticles and Methods of Use Thereof” (patent no. 62,115,968). R.J.L. is inventor of a patent titled “Bacterial Inhibitors” (patent no. 11,504,414).
Figures

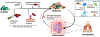
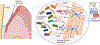
References
-
- Arweiler NB (2021). Oral Mouth Rinses against Supragingival Biofilm and Gingival Inflammation. Monogr Oral Sci 29, 91–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

