COVID-19 vaccination and thyroiditis
- PMID: 36933997
- PMCID: PMC9981269
- DOI: 10.1016/j.beem.2023.101759
COVID-19 vaccination and thyroiditis
Abstract
At the end of 2019, the world began to fight the coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus-2. Many vaccines have quickly been developed to control the epidemic, and with the widespread use of vaccines globally, several vaccine-related adverse events have been reported. This review mainly focused on COVID-19 vaccination-associated thyroiditis and summarized the current evidence regarding vaccine-induced subacute thyroiditis, silent thyroiditis, Graves' disease, and Graves' orbitopathy. The main clinical characteristics of each specific disease were outlined, and possible pathophysiological mechanisms were discussed. Finally, areas lacking evidence were specified, and a research agenda was proposed.
Keywords: COVID-19; Graves’ disease; Hashimoto thyroiditis; SARS-CoV-2; painless thyroiditis; subacute thyroiditis; thyroiditis; vaccination; vaccine.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures

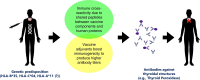
References
-
- WHO Technical Guidance. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It (2020). 2020.
-
- Kojima M., Nakamura S., Oyama T., et al. Cellular composition of subacute thyroiditis. an immunohistochemical study of six cases. Pathol Res Pr. 2002;198:833–837. - PubMed
-
- Pearce E.N., Farwell A.P., Braverman L.E. Thyroiditis. N. Engl J. Med. 2003;348:2646–2655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

