Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: a promising target in anti-cancer therapies
- PMID: 36934087
- PMCID: PMC10024702
- DOI: 10.1038/s41389-023-00460-8
Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: a promising target in anti-cancer therapies
Abstract
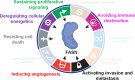
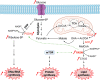
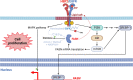
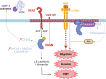
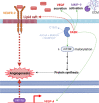
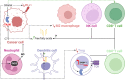
An accelerated de novo lipogenesis (DNL) flux is a common characteristic of cancer cells required to sustain a high proliferation rate. The DNL enzyme fatty acid synthase (FASN) is overexpressed in many cancers and is pivotal for the increased production of fatty acids. There is increasing evidences of the involvement of FASN in several hallmarks of cancer linked to its ability to promote cell proliferation via membranes biosynthesis. In this review we discuss about the implication of FASN in the resistance to cell death and in the deregulation of cellular energetics by increasing nucleic acids, protein and lipid synthesis. FASN also promotes cell proliferation, cell invasion, metastasis and angiogenesis by enabling the building of lipid rafts and consequently to the localization of oncogenic receptors such as HER2 and c-Met in membrane microdomains. Finally, FASN is involved in immune escape by repressing the activation of pro-inflammatory cells and promoting the recruitment of M2 macrophages and T regulatory cells in the tumor microenvironment. Here, we provide an overview of the involvement of the pro-oncogenic enzyme in the hallmarks of cancer making FASN a promising target in anti-cancer therapy to circumvent resistance to chemotherapies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

