CCR3 plays a role in murine age-related cognitive changes and T-cell infiltration into the brain
- PMID: 36934154
- PMCID: PMC10024715
- DOI: 10.1038/s42003-023-04665-w
CCR3 plays a role in murine age-related cognitive changes and T-cell infiltration into the brain
Abstract
Targeting immune-mediated, age-related, biology has the potential to be a transformative therapeutic strategy. However, the redundant nature of the multiple cytokines that change with aging requires identification of a master downstream regulator to successfully exert therapeutic efficacy. Here, we discovered CCR3 as a prime candidate, and inhibition of CCR3 has pro-cognitive benefits in mice, but these benefits are not driven by an obvious direct action on central nervous system (CNS)-resident cells. Instead, CCR3-expressing T cells in the periphery that are modulated in aging inhibit infiltration of these T cells across the blood-brain barrier and reduce neuroinflammation. The axis of CCR3-expressing T cells influencing crosstalk from periphery to brain provides a therapeutically tractable link. These findings indicate the broad therapeutic potential of CCR3 inhibition in a spectrum of neuroinflammatory diseases of aging.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following competing interest: all authors were employees of Alkahest, Inc. at the time of their contribution.
Figures

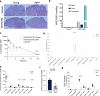
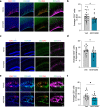
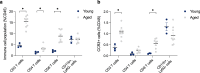

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

