The localization of molecularly distinct microglia populations to Alzheimer's disease pathologies using QUIVER
- PMID: 36934255
- PMCID: PMC10024857
- DOI: 10.1186/s40478-023-01541-w
The localization of molecularly distinct microglia populations to Alzheimer's disease pathologies using QUIVER
Abstract
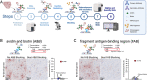
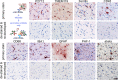
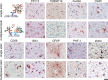
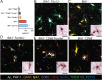
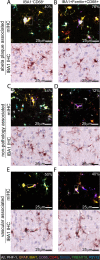
New histological techniques are needed to examine protein distribution in human tissues, which can reveal cell shape and disease pathology connections. Spatial proteomics has changed the study of tumor microenvironments by identifying spatial relationships of immunomodulatory cells and proteins and contributing to the discovery of new cancer immunotherapy biomarkers. However, the fast-expanding toolkit of spatial proteomic approaches has yet to be systematically applied to investigate pathological alterations in the aging human brain in health and disease states. Moreover, post-mortem human brain tissue presents distinct technical problems due to fixation procedures and autofluorescence, which limit fluorescence methodologies. This study sought to develop a multiplex immunohistochemistry approach (visualizing the immunostain with brightfield microscopy). Quantitative multiplex Immunohistochemistry with Visual colorimetric staining to Enhance Regional protein localization (QUIVER) was developed to address these technical challenges. Using QUIVER, a ten-channel pseudo-fluorescent image was generated using chromogen removal and digital microscopy to identify unique molecular microglia phenotypes. Next, the study asked if the tissue environment, specifically the amyloid plaques and neurofibrillary tangles characteristic of Alzheimer's disease, has any bearing on microglia's cellular and molecular phenotypes. QUIVER allowed the visualization of five molecular microglia/macrophage phenotypes using digital pathology tools. The recognizable reactive and homeostatic microglia/macrophage phenotypes demonstrated spatial polarization towards and away from amyloid plaques, respectively. Yet, microglia morphology appearance did not always correspond to molecular phenotype. This research not only sheds light on the biology of microglia but also offers QUIVER, a new tool for examining pathological alterations in the brains of the elderly.
Keywords: Digital pathology; Glia; Histology; Multiplexed tissue imaging; Neurodegenerative disease; Neuropathology; Single-cell analysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG057461/AG/NIA NIH HHS/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- RF1NS119165/NS/NINDS NIH HHS/United States
- R01 AG064234/AG/NIA NIH HHS/United States
- R01NS103785/NS/NINDS NIH HHS/United States
- RF1 NS119165/NS/NINDS NIH HHS/United States
- R01AG064234/AG/NIA NIH HHS/United States
- R21 AG066865/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- R01 NS103785/NS/NINDS NIH HHS/United States
- R01AG068215/AG/NIA NIH HHS/United States
- R21AG066865/AG/NIA NIH HHS/United States
- T32 AG078110/AG/NIA NIH HHS/United States
- R01 AG068215/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

