Clinicopathological and molecular characterization of a case classified by DNA‑methylation profiling as "CNS embryonal tumor with BRD4-LEUTX fusion"
- PMID: 36934287
- PMCID: PMC10024856
- DOI: 10.1186/s40478-023-01549-2
Clinicopathological and molecular characterization of a case classified by DNA‑methylation profiling as "CNS embryonal tumor with BRD4-LEUTX fusion"
Abstract
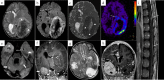
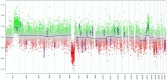
Central Nervous System (CNS) embryonal tumors represent a heterogeneous group of highly aggressive tumors occurring preferentially in children but also described in adolescents and adults. In 2021, the CNS World Health Organization (WHO) classification drastically changed the diagnosis of the other CNS embryonal tumors including new histo-molecular tumor types. Here, we report a pediatric case of a novel tumor type among the other CNS embryonal tumors classified within the methylation class "CNS Embryonal Tumor with BRD4-LEUTX Fusion". The patient was a 4-year girl with no previous history of disease. For a few weeks, she suffered from headaches, vomiting and mild fever associated with increasing asthenia and loss of weight leading to a global deterioration of health. MRI brain examination revealed a large, grossly well-circumscribed tumoral mass lesion located in the left parietal lobe, contralateral hydrocephalus and midline shift. Microscopic examination showed a highly cellular tumor with a polymorphic aspect. The majority of the tumor harbored neuroectodermal features composed of small cells with scant cytoplasm and hyperchromatic nuclei associated with small "medulloblastoma-like" cells characterized by syncytial arrangement and focally a streaming pattern. Tumor cells were diffusely positive for Synaptophysin, CD56, INI1 and SMARCA4 associated with negativity for GFAP, OLIG-2, EMA, BCOR, LIN28A and MIC-2. Additional IHC features included p53 protein expression in more than 10% of the tumor's cells and very interestingly, loss of H3K27me3 expression. The Heidelberg DNA-methylation classifier classified this case as "CNS Embryonal Tumor with BRD4:LEUTX Fusion". RNA-sequencing analyses confirmed the BRD4 (exon 13)-LEUTX (exon 2) fusion with no other molecular alterations found by DNA sequencing. Our case report confirmed that a new subgroup of CNS embryonal tumor with high aggressive potential, loss of H3K27me3 protein expression, BRDA4-LEUTX fusion, named "Embryonal CNS tumor with BRD4-LEUTX fusion", has to be considered into the new CNS WHO classification.
Keywords: BRD4; Brain tumor; CNS embryonal tumor; DNA methylation; H3K27me3 protein expression; LEUTX.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Alexandrescu S, Meredith DM, Lidov HG, Alaggio R, Novello M, Ligon KL, Vargas SO. Loss of histone H3 trimethylation on lysine 27 and nuclear expression of transducin-like enhancer 1 in primary intracranial sarcoma, DICER1-mutant. Histopathology. 2021;78:265–275. doi: 10.1111/his.14217. - DOI - PubMed
-
- Barresi S, Giovannoni I, Rossi S, Stracuzzi A, Quacquarini D, Cafferata B, Piscitelli D, De Leonardis F, Marzullo A, Alaggio R. A novel BRD4–LEUTX fusion in a pediatric sarcoma with epithelioid morphology and diffuse S100 expression. Genes Chromosomes Cancer. 2021;60:647–652. doi: 10.1002/gcc.22974. - DOI - PubMed
-
- Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, Kratz A, Wefers AK, Huang K, Pajtler KW, Schweizer L, Stichel D, Olar A, Engel NW, Lindenberg K, Harter PN, Braczynski AK, Plate KH, Dohmen H, Garvalov BK, Coras R, Hölsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez FJ, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Brück W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hänggi D, Hans V, Rozsnoki S, Hansford JR, Kohlhof P, Kristensen BW, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Mühleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo NG, Driever PH, Kramm CM, Müller HL, Rutkowski S, von Hoff K, Frühwald MC, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu C-M, Perry A, Jones C, Jacques TS, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins VP, Blümcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott PA, Paulus W, Gajjar A, Robinson GW, Taylor MD, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis MA, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schüller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison DW, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, von Deimling A, Pfister SM. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. - DOI - PMC - PubMed
-
- Decock A, Creytens D, Lefever S, Van der Meulen J, Anckaert J, De Ganck A, Deleu J, De Wilde B, Fierro C, Kuersten S, Luypaert M, Rottiers I, Schroth GP, Steyaert S, Vanderheyden K, VandenEynde E, Verniers K, Verreth J, Van Dorpe J, Vandesompele J. mRNA capture sequencing and RT-qPCR for the detection of pathognomonic, novel, and secondary fusion transcripts in FFPE tissue: a sarcoma showcase. Int J Mol Sci. 2022;23:11007. doi: 10.3390/ijms231911007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

