Serum Immune Profiling in Paediatric Crohn's Disease Demonstrates Stronger Immune Modulation With First-Line Infliximab Than Conventional Therapy and Pre-Treatment Profiles Predict Clinical Response to Both Treatments
- PMID: 36934327
- PMCID: PMC10441564
- DOI: 10.1093/ecco-jcc/jjad049
Serum Immune Profiling in Paediatric Crohn's Disease Demonstrates Stronger Immune Modulation With First-Line Infliximab Than Conventional Therapy and Pre-Treatment Profiles Predict Clinical Response to Both Treatments
Abstract
Background: Despite its efficacy, rational guidance for starting/stopping first-line biologic treatment in individual paediatric Crohn's disease [CD] patients is needed. We assessed how serum immune profiles before and after first-line infliximab [FL-IFX] or conventional [CONV] induction therapy associate with disease remission at week 52.
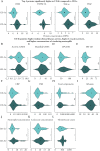
Methods: Pre- [n = 86], and 10-14-week post-treatment [n = 84] sera were collected from patients with moderate-to-severe paediatric CD in the TISKids trial, randomized to FL-IFX [n = 48; five 5-mg/kg infusions over 22 weeks] or CONV [n = 43; exclusive enteral nutrition or oral prednisolone]; both groups received azathioprine maintenance. The relative concentrations of 92 inflammatory proteins were determined with Olink Proteomics; fold changes [FC] with |log2FC| > 0.5 after false discovery rate adjustment were considered significant.
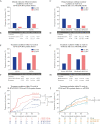
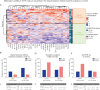
Results: FL-IFX modulated a larger number of inflammatory proteins and induced stronger suppression than CONV; 18/30 proteins modulated by FL-IFX were not regulated by CONV. Hierarchical clustering based on IFX-modulated proteins at baseline revealed two clusters of patients: CD-hi patients had significantly higher concentrations of 23/30 IFX-modulated proteins [including oncostatin-M, TNFSF14, HGF and TGF-α], and higher clinical disease activity, C-reactive protein and blood neutrophils at baseline than CD-lo patients. Only 24% of CD-hi FL-IFX-treated patients maintained remission without escalation at week 52 vs 58% of CD-lo FL-IFX-treated patients. Similarly, 6% of CD-hi CONV-treated patients achieved remission vs 20% of CONV-treated CD-lo patients. Clustering based on immune profiles post-induction therapy did not relate to remission at week 52.
Conclusion: FL-IFX leads to stronger reductions and modulates more immune proteins than CONV. Stratification on pre-treatment profiles of IFX-modulated proteins directly relates to maintenance of remission without treatment escalation.
Trial registration number: NCT02517684.
Keywords: Proteomics; anti-TNF; inflammatory bowel disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
JCE received consultancy fees from Abbvie and Janssen, as well as research support from MSD and Nutricia. LdR: collaboration [e.g. involved in industry-sponsored studies, investigator-initiated study, consultancy] with Abbvie, Lilly, Takeda, Janssen and Pfizer; grant from ZonMw, ECCO, Pfizer. JS, HCR, IT, LMMC, MAC, MMEJ, MvP and YL declare no competing interests.
Figures





References
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L.. Crohn’s disease. Lancet 2017;389:1741–55. - PubMed
-
- Levine A, Chanchlani N, Hussey S, et al. Complicated disease and response to initial therapy predicts early surgery in paediatric Crohn’s disease: Results from the Porto Group Growth Study. J Crohns Colitis 2020;14:71–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

