Fine particulate matter (PM2.5)-induced pulmonary oxidative stress contributes to increases in glucose intolerance and insulin resistance in a mouse model of circadian dyssynchrony
- PMID: 36934930
- PMCID: PMC10164116
- DOI: 10.1016/j.scitotenv.2023.162934
Fine particulate matter (PM2.5)-induced pulmonary oxidative stress contributes to increases in glucose intolerance and insulin resistance in a mouse model of circadian dyssynchrony
Abstract
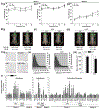
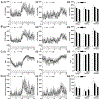
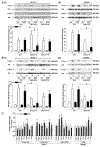
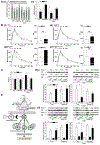
Results of human and animal studies independently suggest that either ambient fine particulate matter (PM2.5) air pollution exposure or a disturbed circadian rhythm (circadian dyssynchrony) are important contributing factors to the rapidly evolving type-2-diabetes (T2D) epidemic. The objective of this study is to investigate whether circadian dyssynchrony increases the susceptibility to PM2.5 and how PM2.5 affects metabolic health in circadian dyssynchrony. We examined systemic and organ-specific changes in glucose homeostasis and insulin sensitivity in mice maintained on a regular (12/12 h light/dark) or disrupted (18/6 h light/dark, light-induced circadian dyssynchrony, LICD) light cycle exposed to air or concentrated PM2.5 (CAP, 6 h/day, 30 days). Exposures during Zeitgeber ZT3-9 or ZT11-17 (Zeitgeber in circadian time, ZT0 = begin of light cycle) tested for time-of-day PM2.5 sensitivity (chronotoxicity). Mice transgenic for lung-specific overexpression of extracellular superoxide dismutase (ecSOD-Tg) were used to assess the contribution of CAP-induced pulmonary oxidative stress. Both, CAP exposure from ZT3-9 or ZT11-17, decreased glucose tolerance and insulin sensitivity in male mice with LICD, but not in female mice or in mice kept on a regular light cycle. Although changes in glucose homeostasis in CAP-exposed male mice with LICD were not associated with obesity, they were accompanied by white adipose tissue (WAT) inflammation, impaired insulin signaling in skeletal muscle and liver, and systemic and pulmonary oxidative stress. Preventing CAP-induced oxidative stress in the lungs mitigated the CAP-induced decrease in glucose tolerance and insulin sensitivity in LICD. Our results demonstrate that circadian dyssynchrony is a novel susceptibility state for PM2.5 and suggest that PM2.5 by inducing pulmonary oxidative stress increases glucose intolerance and insulin resistance in circadian dyssynchrony.
Keywords: Air pollution; Akt phosphorylation; Circadian dyssynchrony; Circadian rhythm; Fine particulate matter, PM(2.5); Insulin resistance; Light pollution; Type 2 diabetes.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, and Hu FB (2003). A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes care 26, 380–384. - PubMed
-
- Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, and Jones DP (2007). Diurnal variation in glutathione and cysteine redox states in human plasma. The American journal of clinical nutrition 86, 1016–1023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

